Purpose

Introduction
Infectious agent
Tick-borne encephalitis (TBE) virus
Endemicity
Focally endemic in the region extending from western and northern Europe to northern and eastern Asia
Traveler categories at greatest risk for exposure and infection
Travelers or expatriates participating in outdoor activities in or near forested areas
Persons consuming unpasteurized dairy products
Prevention methods
Prevent tick bites
Avoid unpasteurized dairy products
Tick-borne encephalitis is a vaccine-preventable disease
Diagnostic support
State health department; or contact CDC Arboviral Diseases Branch (970-221-6400; dvbid@cdc.gov)
Infectious agent
Tick-borne encephalitis (TBE) virus is a single-stranded RNA virus that belongs to the genus Flavivirus. TBE virus has 3 main subtypes: European, Siberian, and Far Eastern.
Transmission
TBE virus is transmitted to humans through the bite of an infected tick of the Ixodes species, primarily Ixodes ricinus (European subtype) or Ixodes persulcatus (Far Eastern and Siberian subtypes). Laboratory experiments suggest the virus can be transmitted from an infected tick to an animal within minutes. Preferred habitats for the tick species are within or near the edges of forests; they favor areas with low-growing dense brush and plant litter. Ticks act as both the vector and virus reservoir. Small rodents are the primary amplifying host. People also can acquire TBE by ingesting unpasteurized dairy products (e.g., milk, cheese) from infected cows, goats, or sheep; transmission from goats is most commonly reported. Infrequently, TBE virus transmission has been reported through laboratory exposure and slaughtering viremic animals. Direct person-to-person spread of TBE virus occurs only rarely, through blood transfusion, solid organ transplantation, or breastfeeding.
Epidemiology
TBE is focally endemic in a geographic region spreading from western and northern Europe to northern and eastern Asia. Approximately 5,000–10,000 TBE cases are reported from endemic countries each year. TBE virus transmission is highly variable by place and over time. Russia, including Siberia, has the most reported cases. The highest disease incidence in recent years has been reported from the Baltic states (Estonia, Latvia, and Lithuania), Czech Republic, and Slovenia. Other European countries with reported cases or known endemic areas are listed in Table 4.18.1. The number of human TBE cases reported from an area might not be a reliable predictor of a traveler's risk for infection because reporting of local cases depends on various factors, including the extent of human and laboratory resources applied to surveillance and the vaccine coverage in the population.
During the past 30 years, the range of TBE virus transmission has expanded to new geographic areas and to higher altitudes. The overall area of recognized transmission has expanded westward and northward, and new TBE virus foci have been detected at elevations up to about 2,100 m (approximately 7,000 ft). These changes are likely due to a complex combination of factors including climatic, ecologic, human, and socioeconomic factors.
Seasonality
The main TBE virus transmission season is April–November when ticks are most active because of warmer weather in the Northern Hemisphere. Peak transmission generally occurs over multiple weeks during the warm, humid summer months, typically during June–August in European countries.
Tick-borne encephalitis among U.S. travelers
The risk for TBE for an individual traveler is greatly affected by their planned itinerary and activities. Most infections result from tick bites acquired in forested areas while participating in activities such as bicycling, birdwatching, camping, fishing, hiking, or collecting berries, flowers, or mushrooms. People with outdoor occupations (e.g., farmers, field researchers, forestry workers, military personnel training in forested areas) are also at increased risk. No U.S. traveler has been reported to have acquired TBE through ingestion of unpasteurized dairy products. TBE risk is negligible for people who remain in urban or unforested areas and who do not consume unpasteurized dairy products.
During 2000–2023, 12 cases of TBE were reported among U.S. civilian travelers. Of these, 11 cases occurred among males, and 9 were in people aged >19 years. Destinations where infections likely were acquired included China, Russia, and several countries in Europe. During 2012–2021, an additional 12 TBE cases were reported among U.S. military personnel or their dependents residing in Germany.
Travelers at risk for TBE virus infection might also be at risk for other tickborne diseases because the same ticks that transmit TBE virus also can transmit other pathogens, including Borrelia burgdorferi (the agent for Lyme disease), Anaplasma phagocytophilum (anaplasmosis), and Babesia spp. (babesiosis); simultaneous infection with multiple organisms has been described.
Table 4.18.1: Country-specific risk information
| Country | Areas with Reported Risk |
|---|---|
| Austria | Highly endemic regions include Carinthia, Styria, Upper Austria, Salzburg, Tyrol, and Vorarlberg Provinces, but transmission foci occur throughout the country |
| Belarus | Almost all of the country considered endemic |
| Belgium | Only a small number of cases reported from geographically dispersed areas |
| Bosnia and Herzegovina | Situation is unclear because limited information is available |
| Bulgaria | Situation is unclear because limited information is available |
| China |
Cases mostly reported from northeastern China, with highest endemicity in Inner Mongolia Autonomous Region (Daxing’an Mountains), Heilongjiang Province (Xiaoxing’an Mountains), and Jilin Province (Changbai Mountains) Other areas with reported transmission include the Xinjiang Autonomous Region (Tianshan and Altai Mountains) and Yunnan and Tibet Provinces A US traveler was previously infected in Tianjin Province |
| Croatia | Highest risk reported in the northeastern and northwestern regions, with lower risk in the central mountainous region, and only sporadic cases reported from the Adriatic coastal region |
| Czech Republic (Czechia) |
Regularly among the countries with the highest reported incidence in Europe Transmission foci occur throughout the country, with greatest risk reported from the Southern Bohemian Region |
| Denmark |
Endemic only on Bornholm, an island in the Baltic Sea Sporadic cases reported from other areas, including from microfoci in North Zealand |
| Estonia |
Regularly among the countries with the highest reported incidence in Europe Transmission foci occur throughout the country, with greatest risk reported from the western part of the country |
| Finland |
Found particularly in some archipelago and coastal areas mostly in the southern part of the country, including municipalities of Kustavi and Pargas and the province of Åland Note: Based on local vaccination recommendations, the greatest risk (as of January 2024) is for persons residing in the Åland Islands, Pargas, Simo, Kemi, Kotka Archipelago, Suvisaaristo Archipelago in Espoo, Sammonlahti District of Lappeenranta, Preiskari Island near Raahe, Kustavi, Sipoo Archipelago, Karhusaari Island in Helsinki, Tohmajarvi, and parts of Lohja (Ojamo, Lylyinen/Hormajarvi, Vohloinen/Virkkala, Kirkniemi), Kirkkonummi (Luoma, Masala), Lappeenranta (Kuusimaki-Lavola), and Raseborg (Bromarv) |
| France |
Main transmission foci are Alsace and the Auvergne-Rhône-Alpes Region Note: French health authorities do not have recommendations for vaccination of the local population |
| Germany |
Highest risk in the southern part of Germany, particularly Baden-Wurttemberg and Bavaria Other key risk areas include southern Hesse, southeastern Thuringia, southeastern Brandenburg, and Saxony Additional localized areas of risk are in central Hesse, Saarland, Rhineland-Palatinate, Lower Saxony, and Saxony-Anhalt |
| Hungary | Highest incidence in the western (Transdanubian) and northern parts of the country |
| Italy | Risk primarily in the pre-alpine and alpine areas in the northeast, including Veneto (mainly Belluno Alps), Friuli-Venezia Giulia, and Trentino-South Tyrol Regions |
| Japan |
Sporadic cases reported from Hokkaido prefecture Note: Japanese health authorities do not have recommendations for vaccination of the local population |
| Kazakhstan | Main risk areas are the Almaty Region, East Kazakhstan Region, and the Sandyktau District of Akmola Region |
| Kyrgyzstan | Situation is unclear because limited information is available. TBE virus-infected ticks reported from Ala-Archa National Nature Park in Tian Shan Mountains |
| Latvia |
Regularly among countries with the highest reported incidence in Europe Transmission foci occur throughout the country, with highest risk in Kurzeme Region in the west and Vidzeme and Riga Regions in central Latvia |
| Liechtenstein | Risk exists in the entire country |
| Lithuania |
Regularly among the countries with highest reported incidence in Europe Transmission foci occur throughout the country |
| Moldova | Situation is unclear because limited information is available |
| Mongolia | Main risk areas are Selenge and Bulgan Provinces |
| Netherlands |
Sporadic cases reported from several different locations Note: Dutch health authorities do not have recommendations for vaccination of the local population |
| Norway | Coastal areas in southern Norway are considered endemic, specifically Agder, Vestfold, and Telemark Counties |
| Poland | Most cases reported from Podlaskie and Warmian-Masurian Provinces, but transmission foci occur throughout the country |
| Romania | Situation is unclear because limited information is available |
| Russia |
Endemic areas widespread across southern part of the non-tropical forest belt High transmission reported from southern parts of Western and Eastern Siberia and Southern Urals |
| Serbia | Situation is unclear because limited information is available |
| Slovakia | Transmission foci occur throughout the country, with higher risk in north and center of the country, particularly Banská Bystrica, Žilina, and Trenčín Regions |
| Slovenia |
Regularly among the countries with highest reported incidence in Europe Transmission foci occur throughout the country, with highest incidence in north and central regions |
| South Korea |
No human cases reported; TBE virus detection in ticks and rodents only Note: South Korean health authorities do not have recommendations for vaccination of the local population |
| Sweden |
Highly endemic in regions around Stockholm (Stockholm Archipelago, Malaren Lake, Uppsala, and Sodermanland Counties) with other main risk areas around lakes in the south (i.e., Vanern, Vattern) Other natural foci in southern and central parts of country |
| Switzerland |
Risk in the entire country, except for cantons of Geneva and Ticino Higher risk in northeastern, central, and midwestern areas of the country, with Thurgau Canton considered highly endemic |
| Tunisia | Situation is unclear because limited information is available and establishment of virus not confirmed |
| Ukraine |
Majority of infections reported from the Volyn Region and Crimea Other foci detected throughout the country, including in the Rivne, Zhytomyr, Kyiv, Chernihiv, and Carpathian Regions |
| United Kingdom |
Small number of cases reported with main risk areas considered to be Thetford Forest, Hampshire/Dorset border, New Forest, and New Yorkshire Moors Note: United Kingdom health authorities do not have recommendations for vaccination of the local population |
Notes
The information should be interpreted cautiously because TBE virus transmission can be highly variable within risk areas and from year to year. Historical data are used to define areas with reported risk and can be incomplete or out of date. More accurate information might be available from national authorities in some countries with TBE transmission risk. If updated information is available, it can be found on the CDC website, Geographic Distribution of Tick-borne Encephalitis.
Clinical presentation
Most (approximately 3 out of 4) infections are asymptomatic. The incubation period for TBE is typically 7–14 days (range, 2–28 days). Acute neuroinvasive disease (i.e., aseptic meningitis, encephalitis, or meningoencephalomyelitis) is the most recognized clinical manifestation of TBE virus infection. Relatively mild forms of disease (e.g., febrile illness) can also occur. TBE can have a monophasic or biphasic course. In patients with a biphasic disease course, the first phase is characterized by non-specific symptoms, including fever and sometimes anorexia, headache, malaise, myalgia, nausea, or vomiting. This phase usually lasts for several days and might be followed by an afebrile and relatively asymptomatic period. The clinical illness in the second phase reflects central nervous system involvement with neurologic symptoms. Clinical findings can include altered mental status, ataxia, cognitive dysfunction, cranial nerve palsies, limb paresis, meningeal signs, rigidity, seizures, or tremors. Children typically present with milder disease and disease severity increases with age. Other risk factors for more severe illness include infection with the Far Eastern subtype or being immunocompromised.
Disease outcome
Outcomes vary by clinical presentation and TBE virus subtype, although some of the reported differences could be due to access to medical care, variations in testing, or methodologic biases in published reports. The European subtype is associated with milder disease, a case-fatality ratio of <2%, and sequelae in 20%–40% of patients overall, including neurologic sequelae such as limb paresis or paralysis in ≤10%. The Far Eastern subtype is often associated with a more severe disease course, with reported case-fatality ratios of 20%–40% among neurologic disease cases. The Siberian subtype has a case-fatality ratio of 6%–8%, with rare reports of cases with slow or chronic progression over months.
Diagnosis
Suspect TBE in travelers who develop a non-specific febrile illness that progresses to neuroinvasive disease ≤4 weeks after arriving from an endemic area. A history of tick bite might suggest TBE diagnosis; however, approximately 30% of patients with TBE do not recall a tick bite.
Serology is typically used for laboratory diagnosis. IgM-capture ELISA performed on serum or cerebrospinal fluid is almost always positive during the neuroinvasive phase of the illness. When interpreting results, consider the patient's vaccination history, date of symptom onset, and information about other flaviviruses known to circulate in the same geographic area that might cross-react in serologic assays.
TBE virus or viral RNA sometimes can be detected during the first phase of illness. By the time neurologic symptoms are recognized, however, the virus or viral RNA is usually undetectable. Therefore, virus isolation and reverse transcription-PCR testing should not routinely be used for diagnosis. However, molecular testing might be preferred in severely immunocompromised persons. Testing for TBE is available at some State Health Departments and CDC. Contact the state or local health department or CDC's Arboviral Diseases Branch, Division of Vector-Borne Diseases (970-221-6400), for assistance with diagnostic testing.
Treatment
No specific antiviral treatment is available for TBE. Management consists of supportive care and treatment of complications.
Prevention
Personal protective measures
Travelers should avoid consuming unpasteurized dairy products and use all measures to avoid tick bites (see Mosquitoes, Ticks, and Other Arthropods chapter).
Vaccine
In 2021, the U.S. Food and Drug Administration approved Pfizer's TICOVAC as the first TBE vaccine for use in the United States. Also marketed as FSME-IMMUN in Europe, TICOVAC is an inactivated, whole-virus vaccine with formulations for children (1–15 years) and adults (≥16 years). Five other TBE vaccines, not licensed for use in the United States, are available internationally.
Indications for use
The risk for most U.S. travelers visiting TBE-endemic areas is very low. Based on activities, location, season, and travel duration, some people who travel abroad are at increased risk for infection (Box 4.18.1). In 2022, the Advisory Committee on Immunization Practices approved recommendations for vaccine use among people traveling or moving to a TBE-endemic area. The vaccine is recommended for persons who will have extensive tick exposure based on planned outdoor activities and itinerary. In addition, TBE vaccine may be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found, with the decision to vaccinate based on an assessment of planned activities and itinerary, risk factors for a poor medical outcome, and personal perception and tolerance of risk. The risk-benefit assessment for vaccination should consider several factors (Box 4.18.2; Figure 4.18.1).
Box 4.18.1
Notes
Abbreviations: TBE, tick-borne encephalitis.
Box 4.18.2
Notes
Abbreviations: TBE, tick-borne encephalitis; TICOVAC, TBE vaccine.
Administration
Dose and primary vaccination schedule vary by age (Table 4.18.2). Each dose is administered intramuscularly. Studies have demonstrated that after a primary series and a booster dose, high seropositivity rates are maintained for at least 10 years in children and healthy adults.
Safety and adverse reactions
Although TICOVAC was only licensed in 2021 in the United States, the vaccine has been available internationally for >20 years, and >75 million doses have been administered with no serious safety concerns identified. Adverse events reported most commonly include tenderness and pain at the injection site in ≥10% of vaccine recipients. In children and adolescents, the most common systemic symptoms include fever and headache, and in adults include headache, fatigue, and myalgia. Serious adverse events are reported only rarely.
Contraindications and precautions
A severe allergic reaction to any component of TICOVAC is a contraindication to administration. Although TBE vaccine is prepared from TBE virus propagated in chick embryo fibroblast cells, the ultracentrifugation process removes all but trace amounts of chick protein, and no reports have been published related to TBE vaccine and egg allergy. However, a known severe hypersensitivity to egg or chicken protein (e.g., anaphylaxis after oral ingestion) is a contraindication to TBE vaccination. Immunocompromise and immunosuppression are precautions to vaccination because some individuals with altered immunocompetence might have reduced immune responses to TICOVAC. No studies have assessed the safety of TICOVAC in women who are pregnant or lactating.
Figure 4.18.1
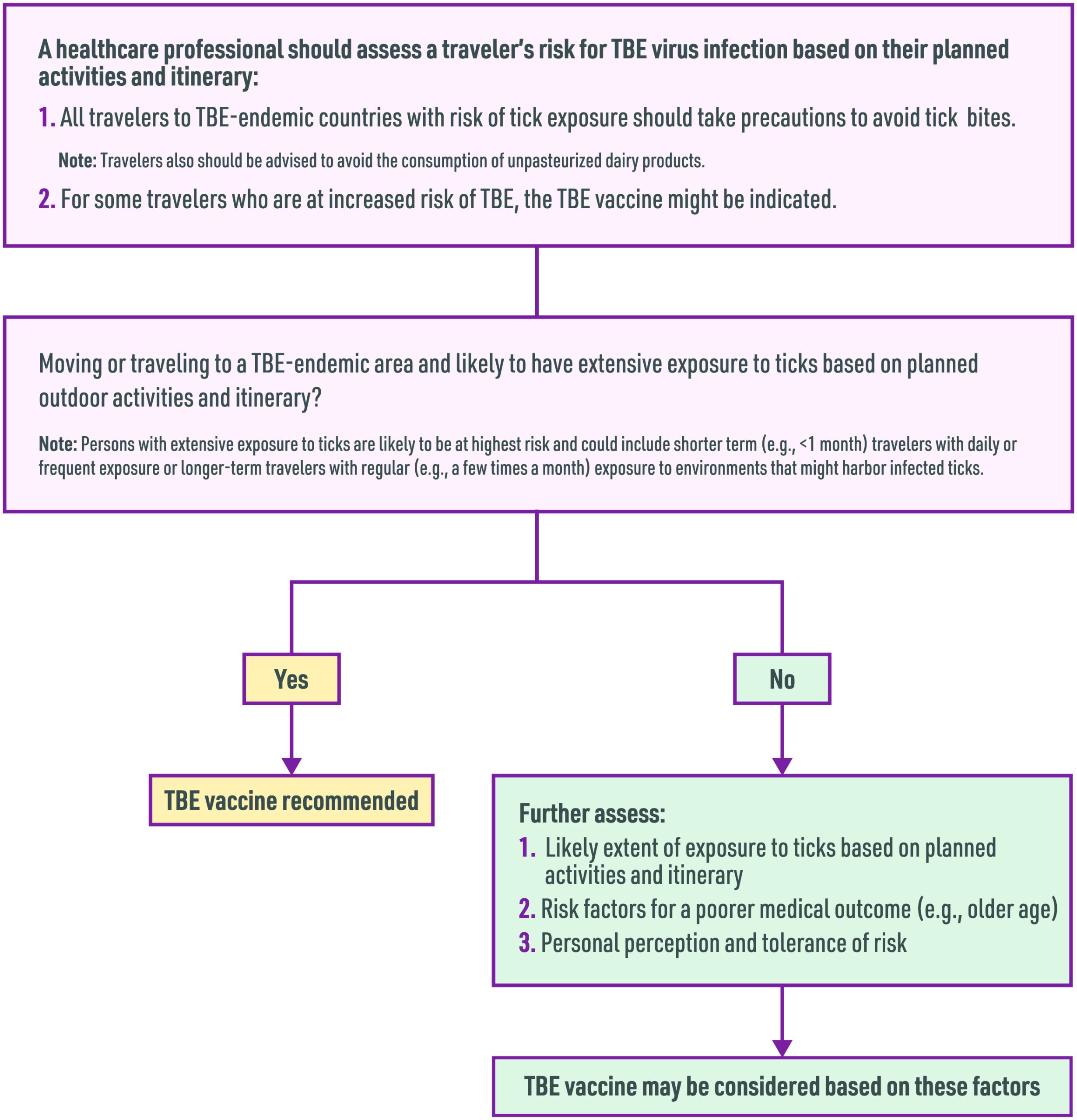
Table 4.18.2: TICOVAC tick-borne encephalitis vaccine administration schedule
| Age | Dose | Primary Vaccination Schedule | Booster |
|---|---|---|---|
| 1–15 years | 0.25 mL |
Dose 1: Day 0 Dose 2: 1–3 months after dose 1 Dose 3: 5–12 months after dose 2 |
≥3 years after completion of primary immunization series if ongoing exposure or re-exposure to TBE virus is expected |
| ≥16 years | 0.5 mL |
Dose 1: Day 0 Dose 2: 14 days–3 months after dose 1 Dose 3: 5–12 months after dose 2 |
≥3 years after completion of primary immunization series if ongoing exposure or re-exposure to TBE virus is expected |
Notes
Abbreviations: TBE, tick-borne encephalitis; TICOVAC, TBE vaccine.
- Centers for Disease Control and Prevention (CDC) (2010). Tick-borne encephalitis among U.S. travelers to Europe and Asia - 2000-2009. MMWR. Morbidity and Mortality Weekly Report, 59(11), 335–338.
- Hills, S. L., Broussard, K. R., Broyhill, J. C., Shastry, L. G., Cossaboom, C. M., White, J. L., Machesky, K. D., Kosoy, O., Girone, K., Klena, J. D., Backenson, B. P., Gould, C. V., Lind, L., Hieronimus, A., Gaines, D. N., Wong, S. J., Choi, M. J., Laven, J. J., Staples, J. E., & Fischer, M. (2022). Tick-borne encephalitis among U.S. travellers, 2010-20. Journal of Travel Medicine, 29(2), taab167. https://doi.org/10.1093/jtm/taab167
- Hills, S. L., Poehling, K. A., Chen, W. H., & Staples, J. E. (2023). Tick-Borne Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR: Recommendations and Reports, 72(5), 1–29. https://doi.org/10.15585/mmwr.rr7205a1
- Mancuso, J. D., Bazaco, S., Stahlman, S., Clausen, S. S., & Cost, A. A. (2019). Tick-borne encephalitis surveillance in U.S. military service members and beneficiaries, 2006-2018. MSMR, 26(11), 4–10.
- Ruzek, D., Avšič Županc, T., Borde, J., Chrdle, A., Eyer, L., Karganova, G., Kholodilov, I., Knap, N., Kozlovskaya, L., Matveev, A., Miller, A. D., Osolodkin, D. I., Överby, A. K., Tikunova, N., Tkachev, S., & Zajkowska, J. (2019). Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antiviral Research, 164, 23–51. https://doi.org/10.1016/j.antiviral.2019.01.014
- Steffen R. (2016). Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. Journal of Travel Medicine, 23(4), taw018. https://doi.org/10.1093/jtm/taw018
- Süss J. (2011). Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks and Tick-Borne Diseases, 2(1), 2–15. https://doi.org/10.1016/j.ttbdis.2010.10.007
- Taba, P., Schmutzhard, E., Forsberg, P., Lutsar, I., Ljøstad, U., Mygland, Å., Levchenko, I., Strle, F., & Steiner, I. (2017). EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. European Journal of Neurology, 24(10), 1214–e61. https://doi.org/10.1111/ene.13356
- Vaccines against tick-borne encephalitis: WHO position paper. (2011). Releve Epidemiologique Hebdomadaire, 86(24), 241–256. https://www.who.int/publications/i/item/WHO-WER8624
