Purpose
Introduction
Infectious agent
Salmonella enterica serotypes Typhi and Paratyphi A, B, and C
Endemicity
Africa
Asia (greatest risk for infection is in South Asia)
Latin America
Traveler categories at greatest risk for exposure and infection
Travelers to low- and middle-income countries where typhoid and paratyphoid fever are endemic
Travelers to mass gatherings
Travelers visiting friends and relatives
Prevention methods
Follow safe food and water precautions
Typhoid fever is a vaccine-preventable disease
Diagnostic support
A clinical laboratory certified in moderate complexity testing; state health department
Infectious agent
Salmonella enterica serotypes Typhi, Paratyphi A, Paratyphi B (tartrate negative), and Paratyphi C cause potentially severe and occasionally life-threatening bacteremic illnesses referred to as typhoid fever (for Typhi serotype) and paratyphoid fever (for Paratyphi serotypes), and collectively as enteric fever. Other Salmonella serotypes, collectively known as nontyphoidal Salmonella, typically cause gastroenteritis as the main symptom.
Transmission
Humans are the only source of the bacteria that cause enteric fever; no animal or environmental reservoirs have been identified. Typhoid and paratyphoid fever are acquired through consumption of water or food contaminated by feces of an acutely infected or convalescent person, or a person with chronic, asymptomatic carriage. Risk for infection is high in low- and middle-income countries with endemic disease, poor access to safe food and water, and poor sanitation. Mass gatherings in endemic areas have been linked to outbreaks of enteric fever. Sexual contact, particularly among men who have sex with men, is a rare route of transmission.
Epidemiology
An estimated 9.2 million cases of typhoid fever and 3.8 million cases of paratyphoid fever occur worldwide each year, causing an estimated 133,000 deaths. In 2019, before the COVID-19 pandemic, approximately 450 culture-confirmed cases of typhoid fever and 130 cases of paratyphoid fever caused by Paratyphi A were reported in the United States; during 2020–2021 when U.S. travel patterns were influenced by COVID-19, there were approximately 150–160 cases of typhoid fever and approximately 30–50 cases of paratyphoid fever caused by Paratyphi A each year. Paratyphoid fever caused by Paratyphi B or Paratyphi C is rarely reported. Approximately 85% of typhoid fever and 80% of paratyphoid fever cases in the United States occur among international travelers; of those, more than 70% are in travelers returning from South Asia, primarily Bangladesh, India, and Pakistan. Other travelers with enteric fever have visited Africa, Latin America, and Southeast Asia, or less commonly, East Asia and the Caribbean.
Travelers visiting friends and relatives are at increased risk because they might be less careful with food and water while abroad than other travelers and might not seek pre-travel health consultation or typhoid vaccination (see Visiting Friends and Relatives: VFR Travel chapter). Although the risk of acquiring illness increases with the duration of stay, travelers have acquired typhoid fever during visits of <1 week to countries where the disease is highly endemic (e.g., Bangladesh, India, Pakistan).
Clinical presentation
The typical incubation period for typhoid fever is 6–30 days, compared with 1–10 days for paratyphoid fever, although the range may vary with host factors and infectious dose. Illness onset is insidious, with gradually increasing fatigue and a fever that increases daily from low-grade to 39°C–40°C (approximately 102°F–104°F) by the third or fourth day of illness. Fever is commonly lowest in the morning, peaking in the late afternoon or evening. Anorexia, headache, and malaise are nearly universal, and abdominal pain, constipation, and diarrhea are common. Diarrhea and vomiting are more common in children than in adults. People also can have dry cough, fatigue, myalgias, and sore throat. Hepatosplenomegaly often can be detected. A transient, maculopapular rash of rose spots can occasionally be seen on the trunk.
The clinical presentation is often confused with malaria. Healthcare professionals should suspect enteric fever in a person with a history of travel to an endemic area who is not responding to antimalarial medication. Untreated, the disease can last for a month, and case-fatality ratios from the pre-antibiotic era have been reported at >10%. By comparison, the case-fatality ratio in patients who receive prompt medical care is usually <1%. Serious complications of typhoid fever occur in 10%–15% of hospitalized patients, generally after 2–3 weeks of illness, and include life-threatening gastrointestinal hemorrhage, intestinal perforation, and encephalopathy. Paratyphoid fever appears to have a case-fatality ratio roughly half that of typhoid fever.
Diagnosis
Typhoid and paratyphoid fever are nationally notifiable diseases in the United States. Healthcare professionals should report cases to their state or local health department. Identification of a domestically acquired case should prompt a public health investigation to prevent other cases.
Blood culture
Patients with typhoid or paratyphoid fever typically have bacteremia; blood culture is therefore the preferred method of diagnosis. A single culture is positive in only approximately 50% of cases, however. Multiple blood cultures increase the sensitivity and might be required for diagnosis. Depending on the blood culture system used, cultures might need to be held and observed for up to 7 days before reporting a negative result. Although bone marrow culture is more invasive (and therefore less commonly performed), it increases the sensitivity to approximately 80% of cases and is relatively unaffected by previous or concurrent antibiotic use. Stool culture is not usually positive during the first week of illness and has less diagnostic sensitivity than blood culture.
Rapid diagnostic tests
Globally, several commercial rapid diagnostic tests for typhoid fever are available, but their sensitivity and specificity are not optimal. The Widal test measures antibody titers; it is unreliable but widely used in low- and middle-income countries because of its low cost. Serologic tests do not distinguish acute from past infection or vaccination and lack specificity; thus, blood culture remains the preferred method to diagnose acute infections.
Clinical diagnosis
Poor sensitivity and specificity of rapid antibody tests and the time it takes to obtain a positive culture mean that the initial diagnosis must often be made clinically. Typhoid and paratyphoid fever are clinically indistinguishable. The combination of risk factors for infection and gradual onset of fever that increases in severity over several days should raise suspicion of enteric fever.
Treatment
Antibiotic therapy shortens the clinical course of enteric fever and reduces the risk of complications and death. Treatment decisions are complicated by high rates of resistance to many antimicrobial agents, and antimicrobial treatment should be guided by susceptibility testing. A thorough travel history can inform empiric treatment choices while awaiting culture results.
Multidrug-resistant infection
Established resistance to older antibiotics (e.g., ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole) has led to these agents being recommended only as alternative antibiotics for infections with known susceptibility. Multidrug-resistant (MDR) Typhi with resistance to all 3 of these antibiotics has been present for decades. Regional estimates for MDR enteric fever range from 9% of isolates in South Asia (2015–2018) to 36%–59% in parts of Africa (2010–2014).
Fluoroquinolones (e.g., ciprofloxacin) are still considered the treatment of choice for fluoroquinolone-susceptible infections in adults. Most Typhi and Paratyphi A infections in the United States are fluoroquinolone-nonsusceptible, however. Most (>85%) fluoroquinolone-nonsusceptible infections have occurred among travelers returning from South Asia, and such infections have been associated with treatment failure or delayed clinical response.
Extensively drug-resistant infection
Before 2018, among all Typhi and Paratyphi A isolates tested by the U.S. Centers for Disease Control and Prevention (CDC) National Antimicrobial Resistance Monitoring System (NARMS), >99% were susceptible to azithromycin and ceftriaxone, based on resistance criteria for Typhi. However, resistance to both agents is now emerging in the United States. In 2016, an outbreak of extensively drug-resistant (XDR) typhoid fever began in Sindh Province, Pakistan; cases among travelers have since been documented globally. These XDR Typhi isolates are typically resistant to ampicillin, ceftriaxone, chloramphenicol, ciprofloxacin, and trimethoprim-sulfamethoxazole but susceptible to azithromycin and carbapenem antibiotics.
The first U.S. cases of XDR typhoid fever associated with travel to Pakistan were diagnosed in 2018. Through 2023, >120 XDR infections had been documented among U.S. residents, including 12 cases among patients who did not travel internationally in the 30 days before illness began. Ceftriaxone resistance also has been identified in Typhi isolates from U.S. travelers returning from Iraq, India, and Afghanistan. Additionally, resistance to azithromycin has been identified among Typhi and Paratyphi strains isolated from patients in Bangladesh, Cambodia, India, Nepal, Pakistan, Saudi Arabia, and the United States.
Empiric and directed treatment
Empiric treatment should be guided by the patient's travel history. For patients with suspected typhoid fever who traveled to Iraq or Pakistan, or who did not travel internationally before their illness began, empirically treat uncomplicated illness with azithromycin and treat complicated illness with a carbapenem. Ceftriaxone and azithromycin remain appropriate empiric treatment options for travelers returning from most other countries.
Once culture results are available, use susceptibility information to guide treatment. Case reports have suggested that patients with XDR Typhi infection who do not improve on a carbapenem alone might benefit from the addition of a second antibiotic (e.g., azithromycin). Updated information about antimicrobial resistance among isolates from U.S. patients with enteric fever in the United States can be found at the NARMS website.
Cases unresponsive to treatment
Patients treated with antimicrobial agents can continue to have fever for 3–5 days, but the maximum temperature generally decreases each day. Patients sometimes feel worse during the first few days after commencing antibiotic treatment. If fever in a person with typhoid or paratyphoid infection does not subside within 5 days of initiating antibiotic therapy, consider treatment with alternative antibiotics or begin looking for a persistent focus of infection (e.g., an abscess or an infection in a bone, joint, or other extraintestinal site).
Relapse, reinfection, and chronic carriage
Relapse, reinfection, and chronic carriage also can occur. Relapse occurs in ≤10% of patients 1–3 weeks after clinical recovery, requiring further antibiotic treatment. An estimated 1%–4% of treated patients become asymptomatic chronic carriers (defined as people who excrete the organism in stool for ≥12 months); a prolonged antibiotic course is usually required to eradicate the organism. Healthcare professionals should follow state and local health department guidance on any follow-up testing required for the patient to return to work, school, or day care.
Prevention
Food and water precautions
Safe food and water precautions and frequent handwashing, especially before meals, are important in preventing both typhoid and paratyphoid fever (see Food and Water Precautions for Travelers chapter). The Advisory Committee on Immunization Practices (ACIP) recommends typhoid immunization for those 2 years and older traveling to areas with a recognized risk of exposure. However, typhoid vaccines are not 100% effective, and a large bacterial inoculum can overwhelm vaccine-induced immunity. Therefore, vaccinated travelers should follow recommended food and water precautions to prevent enteric fever and other infections. No vaccines are available for paratyphoid fever; thus, food and water precautions are the only prevention methods.
Vaccines
Indications
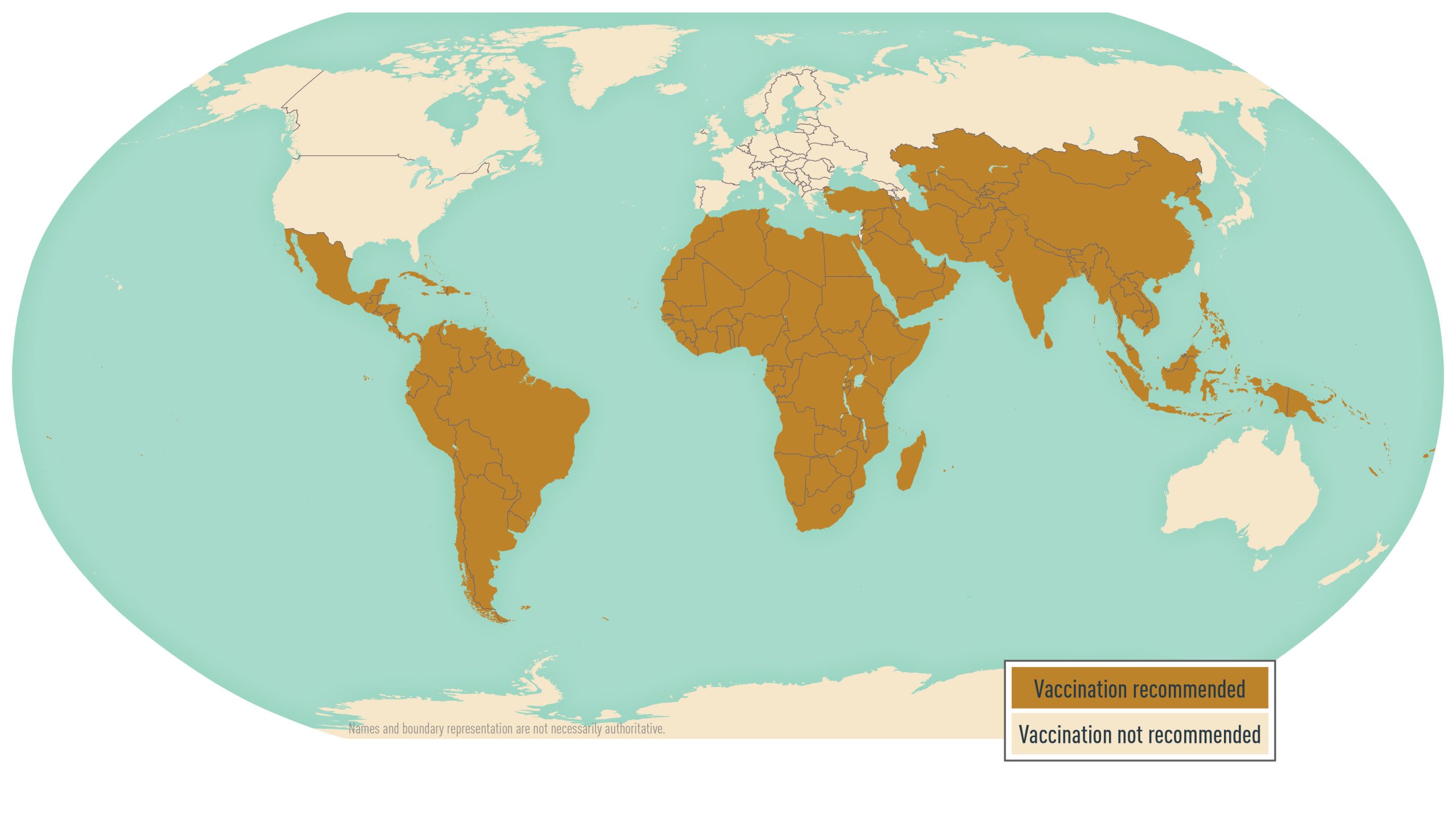
Typhoid vaccine is recommended for travelers 2 years and older going to areas where risk for exposure to Typhi is recognized. Destination-specific vaccine recommendations are available at the CDC Travelers' Health website (see Map 4.20.1). Two typhoid vaccines are licensed for use in the United States: Vi capsular polysaccharide vaccine (ViCPS) (Typhim Vi, manufactured by Sanofi Pasteur) for intramuscular use; and live attenuated vaccine (Vivotif, manufactured from the Ty21a strain of serotype Typhi by PaxVax) for oral use. Both vaccines are unconjugated, which means the polysaccharide antigens are not paired with a protein to elicit a strong response from the immune system. Because these vaccines protect 50%–80% of recipients, remind travelers that typhoid immunization is not 100% effective and take the opportunity to reinforce safe food and water precautions. Neither vaccine is licensed to prevent paratyphoid fever, although limited data from efficacy trials suggest that the Ty21a vaccine might provide some cross-protection against Paratyphi B.
Map 4.20.1
Centers for Disease Control and Prevention
Newer, protein-conjugated Vi vaccines have greater efficacy in children <2 years old and protect people for longer than Vi unconjugated polysaccharide vaccines. Three typhoid Vi conjugate vaccines (TCV) have been licensed in India: Peda Typh (manufactured by Biomed); Typbar-TCV (manufactured by Bharat Biotech); and Zyvac TCV (manufactured by Zydus Cadila). Typbar-TCV also is licensed in Cambodia, Nepal, and Nigeria. As of April 2025, 4 TCVs have been prequalified by the World Health Organization and these vaccines are being introduced into childhood immunization programs in typhoid-endemic countries. TCVs are not currently licensed or available in the United States.
Administration
For information on dosage, administration, and revaccination for the 2 typhoid vaccines licensed in the United States, see Table 4.20.1. The time required for primary vaccination differs, as do the lower age limits for each.
Table 4.20.1: Typhoid fever vaccines licensed in the United States
| Vaccine | Approved Ages for Use | Dose and Route of Administration | Number of Doses | Dosing Interval | Repeat Doses |
|---|---|---|---|---|---|
| VI Capsular polysaccharide vaccine (ViCPS)—Typhim Vi | |||||
| Primary series | ≥2 years | 0.5 mL, IM injection | 1 | N/A | N/A |
| Booster | ≥2 years | 0.5 mL, IM injection | 1 | N/A | Every 2 years |
| Live attenuated Ty21a vaccine—Vivotif1 | |||||
| Primary series | ≥6 years | 1 capsule, orally every other day2 | 4 | 48 hours | N/A |
| Booster | ≥6 years | 1 capsule, orally every other day2 | 4 | 48 hours | Every 5 years |
Notes
Abbreviations: IM, intramuscular; N/A, not applicable.
1Vaccine must be kept refrigerated at 2°C–8°C (35°F–46°F).
2Capsules should be taken with cool liquid, no warmer than 37°C (98.6°F).
Vi capsular polysaccharide vaccine
Primary vaccination with ViCPS consists of one 0.5 mL (25 µg) dose administered intramuscularly ≥2 weeks before travel. The vaccine is approved for use in people ≥2 years old. A dose is recommended every 2 years for those who remain at risk.
Live attenuated Ty21A vaccine
Primary vaccination with Ty21a vaccine consists of 4 capsules, 1 taken every other day. The capsules should be kept refrigerated (not frozen), and all 4 doses must be taken to achieve maximum efficacy. Each capsule should be swallowed whole (not chewed) and taken with cool liquid no warmer than 37°C (98.6°F), approximately 1 hour before a meal and ≥2 hours after a previous meal. Dosing guidelines suggest avoiding alcohol consumption 1 hour before and 2 hours after administration, because alcohol can disintegrate the enteric coating.
Travelers should complete the Ty21a vaccine regimen ≥1 week before potential exposure. The approach for addressing a missed oral vaccine dose or taking a dose late is undefined. Some suggest that minor deviations in the dosing schedule (e.g., taking a dose 1 day late) might not alter vaccine efficacy; no studies have shown the effect of such deviations, however. If travelers do not complete 4 doses as directed, they might not achieve an optimal immune response. The vaccine is approved for use in people ≥6 years old. A booster is recommended every 5 years for those who remain at risk.
Adverse reactions
Adverse reactions most often associated with ViCPS vaccine include headache, injection-site reactions, fever, and general discomfort. Adverse reactions to Ty21a vaccine are rare and mainly consist of abdominal discomfort, diarrhea, fever, headache, nausea, vomiting, and rash. Report adverse reactions to the Vaccine Adverse Event Reporting System by calling 800-822-7967.
Precautions and contraindications
Neither the ViCPS nor the Ty21a vaccine should be given to people with an acute febrile illness; in addition, Ty21a is not recommended for use in people with acute gastroenteritis. Live vaccines, including Ty21a vaccine, should not be given to pregnant or immunocompromised women, including those with HIV. No information is available on the safety of ViCPS in pregnancy; consider ViCPS for pregnant women when the benefits of vaccination outweigh potential risks (e.g., when the likelihood of exposure to Typhi is high).
The intramuscular vaccine (ViCPS) presents a theoretically safer alternative than the live, oral vaccine (Ty21a) for immunocompromised travelers. The Ty21a vaccine can be administered to household contacts of immunocompromised people; although vaccine organisms can be shed transiently in the stool of vaccine recipients, secondary transmission of vaccine organisms has not been documented. The only contraindication to vaccination with ViCPS vaccine is a history of severe local or systemic reactions after a previous dose.
Theoretical concerns have been raised about the immunogenicity of Ty21a vaccine in people concurrently receiving antimicrobial agents, live vaccines, or immune globulin. The growth of the live Ty21a strain is inhibited in vitro by various antimicrobial agents. Vaccination with the Ty21a vaccine should be delayed for >72 hours after the administration of any antimicrobial agent, and antibiotics should not be given to a patient ≤72 hours after the last dose of the Ty21a vaccine. Due to the proguanil component, atovaquone-proguanil should be administered only if 10 days or more have elapsed since the final dose of Vivotif was ingested; mefloquine and chloroquine can be administered together with the Ty21a vaccine.
Ty21a vaccine can be administered simultaneously or at any interval before or after live virus vaccines (e.g., measles-mumps-rubella, oral polio, or yellow fever vaccines). Available data do not suggest that simultaneous administration of live virus vaccines decreases the immunogenicity of the Ty21a vaccine. If typhoid vaccination is warranted, it should not be delayed because of administration of viral vaccines. No data are available on co-administration of the Ty21a vaccine and the current oral cholera vaccine formulation (lyophilized CVD 103-HgR [Vaxchora]). Simultaneous administration of the Ty21a vaccine and immune globulin does not appear to pose a problem.
- Browne, A. J., Kashef Hamadani, B. H., Kumaran, E. A. P., Rao, P., Longbottom, J., Harriss, E., Moore, C. E., Dunachie, S., Basnyat, B., Baker, S., Lopez, A. D., Day, N. P. J., Hay, S. I., & Dolecek, C. (2020). Drug-resistant enteric fever worldwide, 1990 to 2018: a systematic review and meta-analysis. BMC Medicine, 18(1), 1. https://doi.org/10.1186/s12916-019-1443-1
- Crump J. A. (2019). Progress in Typhoid Fever Epidemiology. Clinical Infectious Diseases, 68(Suppl 1), S4–S9. https://doi.org/10.1093/cid/ciy846
- Crump, J. A., Sjölund-Karlsson, M., Gordon, M. A., & Parry, C. M. (2015). Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clinical Microbiology Reviews, 28(4), 901–937. https://doi.org/10.1128/CMR.00002-15
- François Watkins, L. K., Winstead, A., Appiah, G. D., Friedman, C. R., Medalla, F., Hughes, M. J., Birhane, M. G., Schneider, Z. D., Marcenac, P., Hanna, S. S., Godbole, G., Walblay, K. A., Wiggington, A. E., Leeper, M., Meservey, E. H., Tagg, K. A., Chen, J. C., Abubakar, A., Lami, F., Asaad, A. M., Sabaratnam, V., Ikram, A., Angelo, K. M., Walker, A., & Mintz, E. (2020). Update on Extensively Drug-Resistant Salmonella Serotype Typhi Infections Among Travelers to or from Pakistan and Report of Ceftriaxone-Resistant Salmonella Serotype Typhi Infections Among Travelers to Iraq - United States, 2018-2019. MMWR. Morbidity and Mortality Weekly Report, 69(20), 618–622. https://doi.org/10.15585/mmwr.mm6920a2
- Institute for Health Metrics and Evaluation. GBD results from the Global Burden of Disease study. Healthdata.org.https://vizhub.healthdata.org/gbd-results/. Accessed on September 1, 2023.
- Jackson, B. R., Iqbal, S., Mahon, B., & Centers for Disease Control and Prevention (CDC) (2015). Updated recommendations for the use of typhoid vaccine--Advisory Committee on Immunization Practices, United States, 2015. MMWR. Morbidity and Mortality Weekly Report, 64(11), 305–308. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6411a4.htm
- Klemm, E. J., Shakoor, S., Page, A. J., Qamar, F. N., Judge, K., Saeed, D. K., Wong, V. K., Dallman, T. J., Nair, S., Baker, S., Shaheen, G., Qureshi, S., Yousafzai, M. T., Saleem, M. K., Hasan, Z., Dougan, G., & Hasan, R. (2018). Emergence of an Extensively Drug-Resistant Salmonella enterica Serovar Typhi Clone Harboring a Promiscuous Plasmid Encoding Resistance to Fluoroquinolones and Third-Generation Cephalosporins. mBio, 9(1), e00105-18. https://doi.org/10.1128/mBio.00105-18
- McAteer, J., Derado, G., Hughes, M., Bhatnagar, A., Medalla, F., Chatham-Stevens, K., Appiah, G. D., & Mintz, E. (2021). Typhoid Fever in the U.S. Pediatric Population, 1999-2015: Opportunities for Improvement. Clinical Infectious Diseases, 73(11), e4581–e4589. https://doi.org/10.1093/cid/ciaa914
- Syed, K. A., Saluja, T., Cho, H., Hsiao, A., Shaikh, H., Wartel, T. A., Mogasale, V., Lynch, J., Kim, J. H., Excler, J. L., & Sahastrabuddhe, S. (2020). Review on the Recent Advances on Typhoid Vaccine Development and Challenges Ahead. Clinical Infectious Diseases, 71(Suppl 2), S141–S150. https://doi.org/10.1093/cid/ciaa504
