Purpose
Introduction
Infectious agent
Mycobacterium tuberculosis complex
Endemicity
Worldwide but with wide variations by region and social context
Traveler categories at greatest risk for exposure and infection
Humanitarian aid workers and healthcare professionals working in high-prevalence settings (e.g., refugee camps, HIV clinics, and in-patient hospital wards)
Immigrants and refugees
Prevention methods
Avoid high-risk social contexts
Obtain pre- and post-travel testing and treat new infections to prevent tuberculosis disease
Get fit-tested and use respiratory protection (e.g., N95 respirators) in high-risk occupational settings
Consider vaccination with bacillus Calmette-Guérin (no longer available in the United States)
Diagnostic support
A clinical laboratory certified in moderate or high complexity testing; state or local health department; also, consult with U.S. TB Centers of Excellence for Training, Education, and Medical Consultation
Infectious agent
Mycobacterium tuberculosis complex is a group of closely related rod-shaped, non-motile, slow-growing, acid-fast bacteria, which includes Mycobacterium bovis and M. tuberculosis hominis, the most common cause of human tuberculosis (TB), usually referred to as M. tuberculosis.
Transmission
TB transmission occurs when a patient with a contagious form of the infection coughs, spreading bacilli through the air. People can acquire bovine TB (caused by M. bovis) by consuming unpasteurized dairy products from infected cattle or, rarely, by percutaneous or respiratory exposure to other infected mammals.
The risk for M. tuberculosis transmission on an airplane is low, but instances of in-flight TB transmission have occurred. The risk of transmission is dependent on the contagiousness of the person with TB, seating proximity, flight duration, and host factors. To prevent transmission, people with contagious TB should not travel by commercial airplanes or other commercial conveyances. Typically, only TB of the lung or airway is contagious in community contexts, and health department authorities determine whether TB is contagious based on a person's chest radiograph, sputum tests, symptoms, and treatment received. The World Health Organization (WHO) issued guidelines for notifying passengers potentially exposed to TB on airplanes. Passengers concerned about possible TB exposure should see their primary healthcare professional or visit their local health department clinic for evaluation. Healthcare professionals should notify their local health authorities of any patient with contagious TB who traveled or intends to travel by aircraft. Some of the concerns about travel and contagious TB are applicable to other public conveyances, such as cruise ships and long-distance buses.
Bovine TB is a risk for travelers who consume unpasteurized dairy products in countries (e.g., Mexico) where M. bovis in cattle is common. M. bovis risk in some African countries has been postulated, but human M. bovis statistics are unavailable for those countries.
Epidemiology
According to the WHO, approximately 10.6 million new TB cases and 1.3 million TB-related deaths occurred in 2022. The number of cases decreased from 2019 through 2021 while the number of deaths increased during the COVID-19 pandemic. However, in 2022, the number of cases rebounded in the majority of countries to at least 2019 levels and deaths decreased. TB occurs throughout the world, but the incidence varies (Map 4.19.1). In some countries in Sub-Saharan Africa and Asia, the annual incidence is several hundred per 100,000 population. In the United States, the annual incidence is <3 per 100,000 population, but immigrants from countries with a high TB burden and long-term residents of high-burden countries have a 10 times greater incidence of TB than the U.S. national average. Of note, U.S. surveillance does not capture travel-related cases of TB.
Drug-resistant TB is an increasing concern. Multidrug-resistant (MDR) TB is resistant to at least the 2 most effective drugs, isoniazid and rifampin. Extensively drug-resistant TB (XDR TB) is resistant to rifampin, plus any fluoroquinolone, plus at least 1 of either bedaquiline or linezolid. Pre-XDR TB is resistant to rifampin and any fluoroquinolone. Drug-resistant TB is less common than drug-susceptible TB, but globally approximately 410,000 cases of rifampin-resistant TB occurred in 2022, which accounted for >25% of TB cases in some countries (Table 4.19.1). MDR, pre-XDR, and XDR TB are of particular concern among HIV-infected and other immunocompromised people.
Map 4.19.1
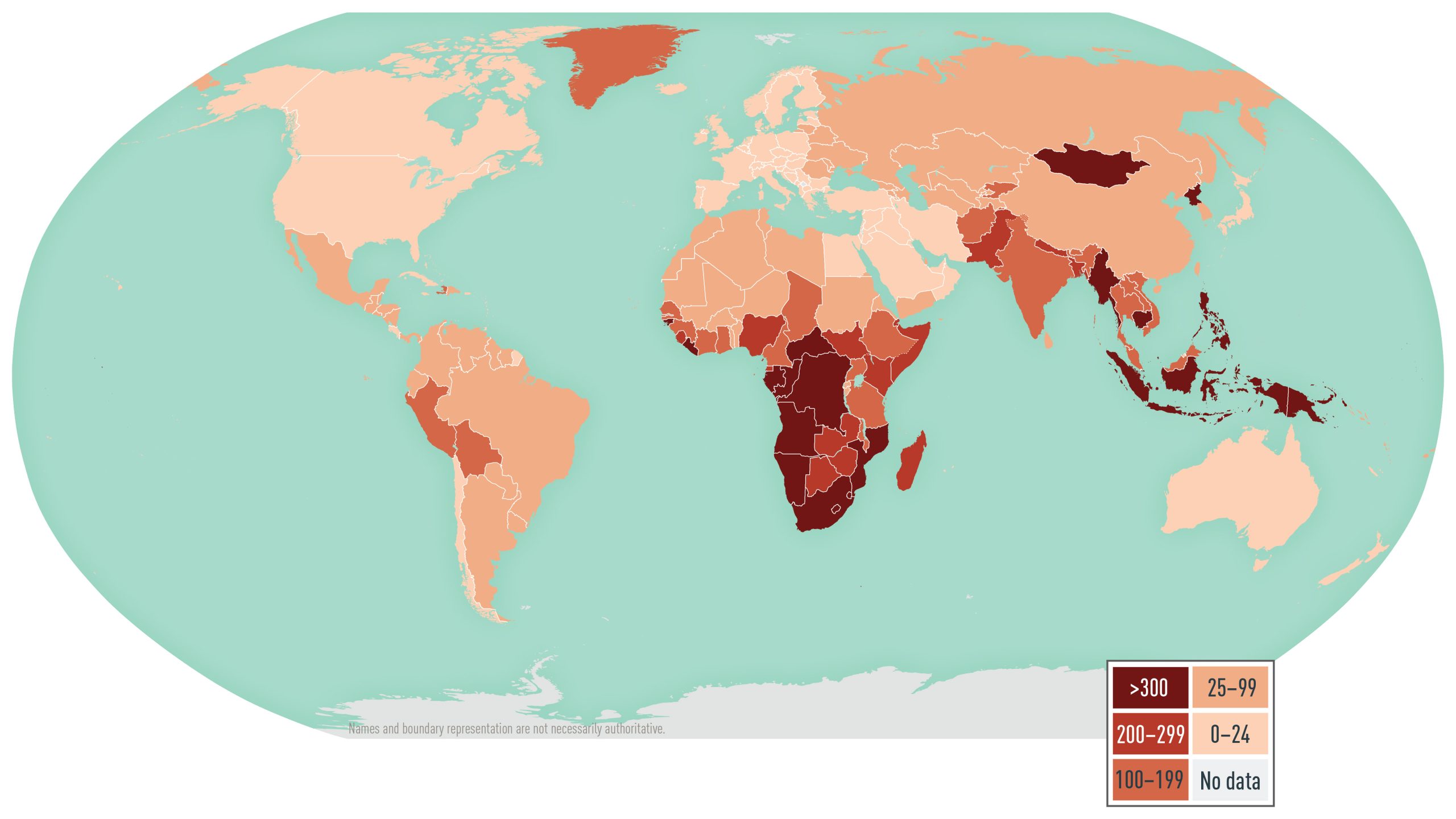
Disease data sources: World Health Organization. Global Tuberculosis Report 2023 (https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1).
Notes
Disease data sources: World Health Organization. Global tuberculosis report 2023 (https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1); for French Guiana, Tableau 5: Taux de déclaration de tuberculose maladie par Nouvelles régions (taux pour 100 000), France entière, 2016–2022; Santé publique France; La tuberculose: données (Table 5: Tuberculosis disease reporting rate by New regions [rate per 100,000], Whole France, 2016–2022, in Public Health France, Tuberculosis: data; https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/tuberculose/donnees/#tabs [Excel]); for Taiwan, Statistics of Communicable Diseases and Surveillance Report 2022, Taiwan Centers for Disease Control, Ministry of Health and Welfare, R.O.C. (Taiwan), December 2023.
Table 4.19.1: Estimated proportion of multidrug-resistant (MDR) or rifampin-resistant (RR) tuberculosis (TB) cases in countries, in 2022, listed by the World Health Organization as having high MDR/RR TB burden in the period 2021–2025
| Country | % of New TB Cases That Are MDR/RR TB | Country | % of New TB Cases That Are MDR/RR TB |
|---|---|---|---|
| Angola | 4% | Pakistan | 2.3% |
| Azerbaijan | 12% | Papua New Guinea | 3.6% |
| Bangladesh | 1.1% | Peru | 4.9% |
| Belarus | 40% | Philippines | 2.5% |
| China | 3% | Republic of Moldova | 28% |
| DPR Korea | 2.4% | Russian Federation | 37% |
| DR Congo | 1.6% | Somalia | 4.6% |
| India | 2.5% | South Africa | 2.9% |
| Indonesia | 2.2% | Tajikistan | 28% |
| Kazakhstan | 34% | Ukraine | 29% |
| Mongolia | 7.2% | Uzbekistan | 16% |
| Mozambique | 2.9% | Vietnam | 4.5% |
| Myanmar | 4.1% | Zambia | 2.2% |
| Nepal | 4% | Zimbabwe | 1.7% |
| Nigeria | 2.1% |
Notes
Abbreviations: MDR, multidrug-resistant; RR, rifampin-resistant, TB, tuberculosis; WHO, World Health Organization.
Global Tuberculosis Report 2023, Figure A3.1 of ANNEX 3, WHO website.
Clinical presentation
M. tuberculosis infection can be detected by a positive tuberculin skin test (TST) or interferon-gamma release assay (IGRA) 8–10 weeks after exposure; however, these tests are not sufficiently sensitive for excluding TB disease reliably. Overall, 5%–10% of otherwise healthy people who are infected will have progression to TB disease during their lifetimes. Progression to TB disease can take weeks to decades after initial infection; the greatest likelihood of progression is in the first 2 years. Infants and people with immune systems that are impaired by medical conditions or treatments are more likely to have early, rapid progression. For example, progression is 8%–10% per year in HIV-infected people not receiving antiretroviral therapy. People receiving tumor necrosis factor blockers to treat rheumatoid arthritis and other chronic inflammatory conditions also are at increased risk for disease progression. People with TB disease have symptoms or other manifestations of illness (e.g., an abnormal chest radiograph). For most people who become infected, M. tuberculosis remains in an inactive state (latent TB infection, or LTBI) in which the infected person has no symptoms and cannot spread the infection to others.
TB disease can affect any organ, but it affects the lungs in 70%–80% of cases. Pulmonary TB symptoms include prolonged cough, fever, hemoptysis, night sweats, decreased appetite, and weight loss. The most common sites for TB outside the lungs (i.e., extrapulmonary TB) are the bladder, bones and joints, brain and meninges, genitalia, kidneys, lymph nodes, and pleura.
Diagnosis
Pre- and post-travel testing for Mycobacterium tuberculosis infection
Before leaving the United States, travelers who anticipate possible prolonged exposure to TB (e.g., people who will care for patients, or who will work in healthcare facilities, prisons or jails, refugee camps, or homeless shelters) and those planning prolonged stays in places with high rates of TB-transmission should have a pre-travel test for M. tuberculosis infection (e.g., QuantiFERON-TB Gold Plus, T-SPOT.TB, 2-step tuberculin skin test [TST]).
Screening for asymptomatic M. tuberculosis infections before and after travel should be carried out for travelers at risk of acquiring TB at their destinations. Screening with a TST or an IGRA in travelers at very low risk might produce false-positive test results, leading to unnecessary additional examinations or treatment. IGRAs, which require a single blood draw, are approximately as specific as TST in people who have not been vaccinated with bacillus Calmette-Guérin (BCG) and are more specific in BCG-vaccinated people. BCG is administered as a single dose shortly after birth in the majority of countries but not in the United States. In western European countries, Canada, Australia, and New Zealand, BCG is used selectively for groups who have persistent TB problems.
TST is prone to boosting of sensitivity in serial testing, necessitating a 2-step initial test for a baseline, which is unneeded with IGRAs. The first TST can restore the ability to react to subsequent tests, resulting in a "booster" reaction. None of the general factors that increase the likelihood of boosting, such as older age, are reliable for deciding whether 2-step initial testing with TST is necessary. If TST is used for pre-travel testing, use the 2-step TST for any traveler undergoing TST testing for the first time. The 2-step method is not needed for travelers who have already been tested and found to have a negative result within the previous 2 years. For the 2-step method, someone whose baseline TST yields a negative result should be retested 1–3 weeks after the initial test; if the second test result is negative, the patient can be considered not infected. If the second test result is positive, the patient is classified as having skin test boosting— the possible causes are previous M. tuberculosis infection with waning sensitization, BCG vaccination, or infection with non-tuberculous mycobacteria. Without checking for boosting, a future positive result could be misinterpreted as a new M. tuberculosis infection (recent conversion) rather than a boosted reaction. For travelers who do not have enough time to complete a 2-step TST before departure, an IGRA is preferred over a single-step TST.
Using TST or an IGRA in very-low-prevalence populations will probably produce more false positives than true positives. Pre-travel testing should be limited to people who are likely to be exposed to contagious TB while overseas, including those going to live in places with high rates of TB-transmission or anyone intending to spend any length of time in routine contact with patients in healthcare facilities or populations living in congregate settings (e.g., homeless shelters, prisons, refugee camps). People at low risk for exposure to TB, who comprise most travelers, do not need to be screened before or after travel. If an IGRA is used for pre-travel testing and there is concern for a false-positive result in a traveler otherwise considered to be at low risk, a second test can be used, which confirms TB infection only if both tests are positive.
CDC guidelines recommend an IGRA (as opposed to TST) for people aged ≥5 years in populations at low risk. IGRA is not recommended for younger children in CDC guidelines because of limited study data with comparisons between TST and an IGRA in young children. Based on observational data and expert opinion, the American Academy of Pediatrics guidelines recommend an IGRA for children ≥2 years old; some pediatric TB experts use IGRAs for all children.
If the result of a pre-travel test (either IGRA or 2-step TST) for M. tuberculosis infection is negative, a traveler should have a post-travel test with the same type of test used pre-travel, 8–10 weeks after returning from their trip. The waiting period of 8–10 weeks allows for sensitization to develop after recent infection. The use of the same test type facilitates interpretation of results. The U.S. Food and Drug Administration (FDA) has approved 2 commercially available tuberculin solutions for skin testing: Aplisol (JHP Pharmaceuticals) and Tubersol (Sanofi Pasteur). People who have repeat TSTs should be tested with the same tuberculin purified protein derivative solution because switching products can lead to different test results. During extended (>6 months) stays in, or repeated travel to, high-risk settings, travelers should have repeat testing every 6–12 months while traveling outside the United States and then 8–10 weeks after final return, all with the same type of test used pre-travel.
In general, do not mix the types of tests used for a person. The discordance rate between TST and IGRA results is as much as 15%; in most instances of discordance, the TST result is positive and the IGRA is negative. Healthcare professionals cannot be confident about the reason for discordance in any single person. If a healthcare professional does decide to mix tests, going from TST to IGRA is better than the other way around, because the likelihood of a discordant result with the TST negative and the IGRA positive is lower.
For travelers who were born or took up residence in places with high rates of TB-transmission, consider the greater background prevalence of infection in these places. In a study among 53,000 adults in Tennessee, the prevalence of a positive TST result among participants born outside the United States was >11 times that of U.S.-born participants (34% vs 3%). Confirming M. tuberculosis test status before travel would prevent the conclusion that a positive result after travel was due to recent infection.
Travelers who suspect they have been exposed to TB should inform their healthcare professional of the possible exposure and receive a medical evaluation. People with HIV infection or other immunocompromising conditions are more likely to have an impaired response to TST or an IGRA; be sure to ask travelers about such underlying conditions. Because TB with drug resistance is relatively common in some parts of the world, consult with experts in infectious diseases or pulmonary medicine regarding proper management and coordinate consultations with input from the public health department.
Diagnostic testing recommendations for tuberculosis disease
In 2017, CDC, the American Thoracic Society (ATS), and the Infectious Diseases Society of America (IDSA) jointly published diagnostic recommendations. Collect sputum or other respiratory specimens for culture and smears for acid-fast bacilli (AFB) from people being examined for pulmonary TB.
Although diagnosis of TB disease can be made using clinical criteria in the absence of microbiologic confirmation, perform laboratory testing to confirm the diagnosis, guide treatment decisions, and provide bacterial DNA for molecular epidemiology. Molecular tests for mutations that confer drug resistance can be performed directly on specimens and can guide initial treatment while culture results are pending. Culture-based susceptibility testing is recommended for all patients with a positive culture result to help determine the appropriate drug regimen.
Culture methods
Culture methods, with referral to a public health reference laboratory in some instances, are necessary to identify the M. tuberculosis complex species responsible for infection. Culture and identification of M. tuberculosis takes approximately 2–8 weeks.
Microscopy
A preliminary diagnosis of TB can be made when AFB are seen by microscopy on a sputum smear or in other body tissues or fluids. Microscopy cannot distinguish M. tuberculosis from non-tuberculous mycobacteria, however, which is particularly problematic in countries like the United States, where the prevalence of infection with non-tuberculous mycobacteria is greater than that of TB.
Nucleic acid amplification tests
Less sensitive than culture but more sensitive than AFB smear and more rapid than culture methods, nucleic acid amplification tests (NAATs) are specific for the M. tuberculosis complex. NAATs detect all members of the M. tuberculosis complex. Thus, a positive NAAT result can rapidly confirm a diagnosis and help guide initial treatment until culture results return. U.S. diagnostic guidelines suggest performing an NAAT on the initial respiratory specimen from persons being evaluated for having pulmonary TB.
The availability of NAATs and the policies for ordering these tests are locally determined, and healthcare professionals should consult TB control officials at their state health department. Diagnosis of extrapulmonary TB disease can be confirmed with an NAAT positive for M. tuberculosis complex if the test has been validated for the specimen type or with a culture positive for M. tuberculosis from affected body tissues or fluids.
Diagnostic support
TB disease is a nationally notifiable condition in the United States. LTBI is also notifiable in many jurisdictions. LTBI is diagnosed by a positive result from an IGRA or TST after further examinations (e.g., chest radiograph, symptom review) have excluded TB disease.
Expertise in the diagnosis of TB and its specialty laboratory services, or local referral for such expertise, is available from the health departments of cities, counties, and states. In most settings, contact tracing is managed by public health officials. General information and expert medical consultation also are available from the CDC-sponsored U.S. TB Centers of Excellence for Training, Education, and Medical Consultation.
Treatment
Latent tuberculosis infection
People with LTBI can be treated, and treatments are effective at preventing progression to TB disease. Healthcare professionals must exclude TB disease before starting LTBI treatment. In the United States, several regimens are recommended for the treatment of drug-susceptible LTBI, including 3 months of once-weekly isoniazid and rifapentine; 4 months of daily rifampin; 3 months of daily isoniazid and rifampin; and 6–9 months of daily isoniazid. Given the low completion rates of the 6- to 9-month isoniazid regimen, shorter duration regimens are preferred.
Choose a regimen for patients that is based on coexisting medical conditions, potential for drug interactions, and drug-susceptibility results of the presumed source of exposure, if known. For example, rifampin has interactions with oral contraceptives and certain antiretroviral medications taken by people with HIV/AIDS. Although direct observation of therapy is not required, people who are at especially high risk for TB disease, who might have difficulty adhering to treatment, or who are given an intermittent dosing regimen, are likely candidates for directly observed therapy for LTBI. Standard regimens are recommended if the drug resistance for the source of the infection is unknown. The treatment regimen for latent infection with M. tuberculosis that is believed to be drug resistant because the presumed source is drug resistant should be determined in consultation with a TB expert.
Tuberculosis disease
In 2025, ATS/CDC/ERS/IDSA published consolidated guidelines for treating TB disease. All regimens include multiple drugs administered by directly observed therapy. The standard 6-month regimen for drug-susceptible TB in adults and children is isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months, then isoniazid and rifampin for an additional 4 months. A 4-month regimen of isoniazid, rifapentine, pyrazinamide, and moxifloxacin can be considered for people age 12 years and older. When non-severe TB in children is presumed to be susceptible to isoniazid and rifampin, it can be treated with a 4-month regimen of isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months, then isoniazid and rifampin for an additional 2 months.
Drug-resistant TB is more difficult to treat, historically requiring 18–24 months of 4–6 drugs including injectables. More recently, for persons aged 14 years and older who have rifampin-resistant or multidrug-resistant pulmonary TB, a 6-month all-oral regimen of bedaquiline, pretomanid, and linezolid is recommended, with moxifloxacin added if the TB is fluoroquinolone-susceptible. Treatment of drug-resistant TB should be guided by susceptibility results and is best managed by an expert.
Prevention
Travelers should avoid exposure to people with TB disease in crowded and enclosed environments (e.g., healthcare facilities, prisons or jails, and homeless shelters). Advise travelers who will be caring for patients, or who will be working in healthcare facilities where people with TB are likely to be patients, to consult infection control or occupational health experts about baseline TB infection screening, procedures for obtaining personal respiratory protective devices (e.g., N95 respirators), and recommendations for respirator selection and training.
Based on WHO recommendations, BCG vaccine is administered once, at birth, in countries with higher TB burdens to reduce the severe consequences of TB in infants and children. BCG vaccine has low and variable efficacy in preventing TB in adults. Some experts have advocated vaccinating healthcare professionals likely to be exposed to drug-resistant TB in settings where infection-control measures like those recommended in the United States are not fully implemented. The FDA-approved BCG Live (TICE, Organon Teknika) vaccine has not been available in the United States since 2019. All people, including those who have received BCG vaccination, must follow recommended TB infection-control precautions to the greatest extent possible. An IGRA is preferred over TST for pre-travel and post-travel testing in those vaccinated with BCG because BCG might induce false-positive TST results. No BCG effects on IGRA results have been detected in multiple studies.
To prevent infections from M. bovis and other foodborne pathogens, travelers should avoid consuming unpasteurized dairy products.
- Brown, M. L., Henderson, S. J., Ferguson, R. W., & Jung, P. (2015). Revisiting tuberculosis risk in Peace Corps Volunteers, 2006-13. Journal of Travel Medicine, 23(1), 10.1093/jtm/tav005. https://doi.org/10.1093/jtm/tav005
- Carr, W., Kurbatova, E., Starks, A., Goswami, N., Allen, L., & Winston, C. (2022). Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the Treatment of Drug-Susceptible Pulmonary Tuberculosis - United States, 2022. MMWR. Morbidity and Mortality Weekly Report, 71(8), 285–289. https://doi.org/10.15585/mmwr.mm7108a1
- Centers for Disease Control and Prevention. Provisional CDC Guidance for the Use of Pretomanid as Part of a Regimen [Bedaquiline, Pretomanid, And Linezolid (Bpal)] To Treat Drug-Resistant Tuberculosis Disease. CDC.gov. https://www.cdc.gov/tb/hcp/treatment/bpal.html
- Conradie, F., Diacon, A. H., Ngubane, N., Howell, P., Everitt, D., Crook, A. M., Mendel, C. M., Egizi, E., Moreira, J., Timm, J., McHugh, T. D., Wills, G. H., Bateson, A., Hunt, R., Van Niekerk, C., Li, M., Olugbosi, M., Spigelman, M., & Nix-TB Trial Team (2020). Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. The New England Journal of Medicine, 382(10), 893–902. https://doi.org/10.1056/NEJMoa1901814
- Lewinsohn, D. M., Leonard, M. K., LoBue, P. A., Cohn, D. L., Daley, C. L., Desmond, E., Keane, J., Lewinsohn, D. A., Loeffler, A. M., Mazurek, G. H., O'Brien, R. J., Pai, M., Richeldi, L., Salfinger, M., Shinnick, T. M., Sterling, T. R., Warshauer, D. M., & Woods, G. L. (2017). Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical Infectious Diseases, 64(2), 111–115. https://doi.org/10.1093/cid/ciw778
- Nahid, P., Dorman, S. E., Alipanah, N., Barry, P. M., Brozek, J. L., Cattamanchi, A., Chaisson, L. H., Chaisson, R. E., Daley, C. L., Grzemska, M., Higashi, J. M., Ho, C. S., Hopewell, P. C., Keshavjee, S. A., Lienhardt, C., Menzies, R., Merrifield, C., Narita, M., O'Brien, R., Peloquin, C. A., Raftery, A., Saukkonen, J., Shaaf, S. H., Sotgiu, G., Starke, J. R., Migliori, G. B., & Vernon, A. (2016). Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical Infectious Diseases, 63(7), e147–e195. https://doi.org/10.1093/cid/ciw376
- Seaworth, B. J., Armitige, L. Y., Aronson, N. E., Hoft, D. F., Fleenor, M. E., Gardner, A. F., Harris, D. A., Stricof, R. L., & Nardell, E. A. (2014). Multidrug-resistant tuberculosis. Recommendations for reducing risk during travel for healthcare and humanitarian work. Annals of the American Thoracic Society, 11(3), 286–295. https://doi.org/10.1513/AnnalsATS.201309-312PS
- Sosa, L. E., Njie, G. J., Lobato, M. N., Bamrah Morris, S., Buchta, W., Casey, M. L., Goswami, N. D., Gruden, M., Hurst, B. J., Khan, A. R., Kuhar, D. T., Lewinsohn, D. M., Mathew, T. A., Mazurek, G. H., Reves, R., Paulos, L., Thanassi, W., Will, L., & Belknap, R. (2019). Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR. Morbidity and Mortality Weekly Report, 68(19), 439–443. https://doi.org/10.15585/mmwr.mm6819a3
- Sterling, T. R., Njie, G., Zenner, D., Cohn, D. L., Reves, R., Ahmed, A., Menzies, D., Horsburgh, C. R., Jr, Crane, C. M., Burgos, M., LoBue, P., Winston, C. A., & Belknap, R. (2020). Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR. Recommendations and Reports, 69(1), 1–11. https://doi.org/10.15585/mmwr.rr6901a1
- World Health Organization. Global Tuberculosis Report 2023. WHO.int. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024
