Purpose
Introduction
Infectious agent
Neisseria meningitidis
Endemicity
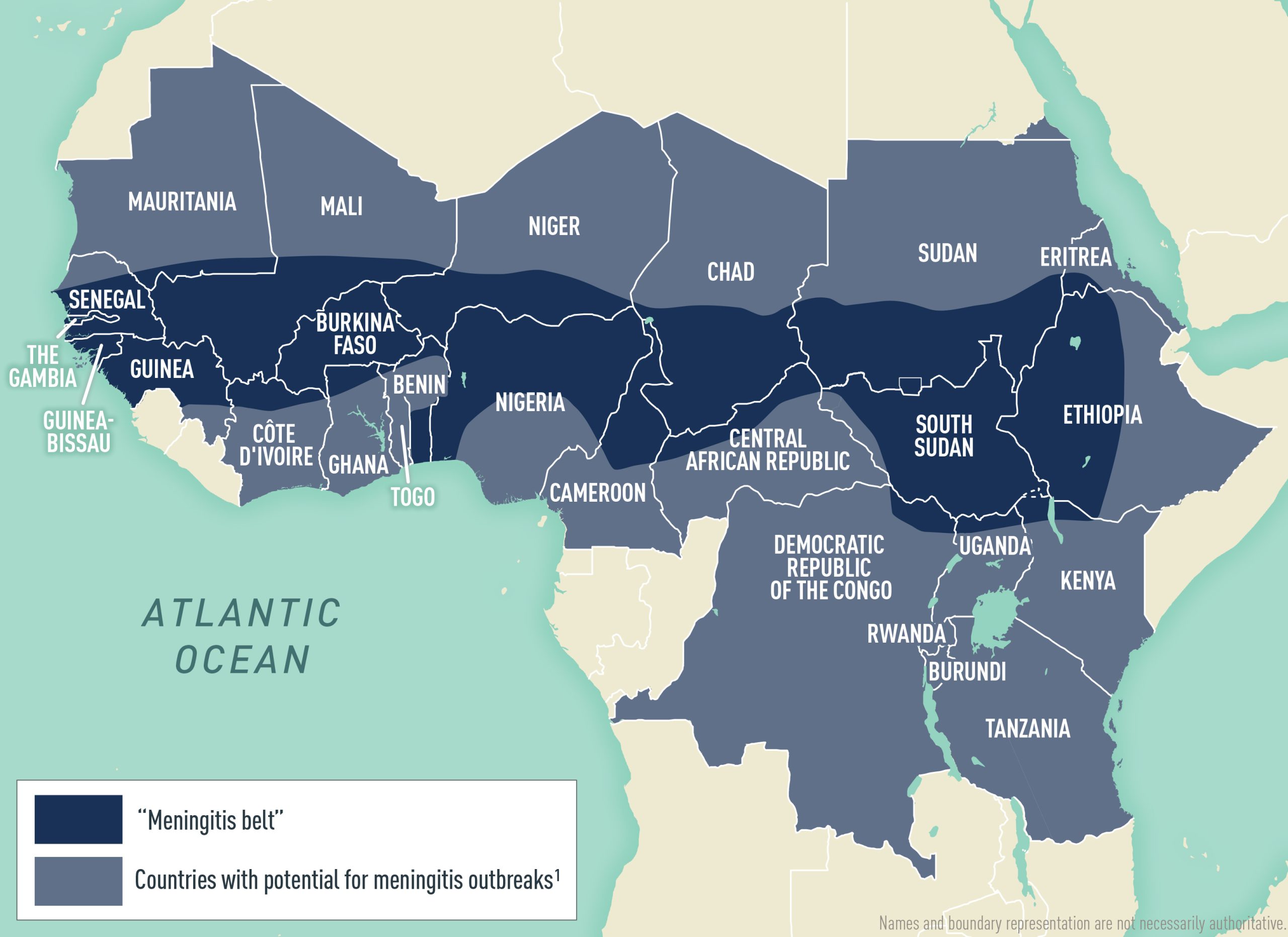
Worldwide, but greatest incidence occurs in the meningitis belt of Africa (Map 4.12.1)
Traveler categories at greatest risk for exposure and infection
Unvaccinated travelers to countries in the meningitis belt, particularly travelers having prolonged contact with local populations during an epidemic
Prevention methods
Most types of meningococcal disease are vaccine-preventable
Diagnostic support
A clinical laboratory certified in moderate complexity testing; state health department
Infectious agent
Neisseria meningitidis is a gram-negative diplococcus bacterium. Meningococci are classified into serogroups based on the composition of their capsular polysaccharide. The 6 major meningococcal serogroups associated with disease are A, B, C, W, X, and Y.
Transmission
Meningococci spread through respiratory secretions and require close contact for transmission. Both asymptomatic carriers and people with overt meningococcal disease can be sources of infection. Asymptomatic carriage is transient and typically affects approximately 5%–10% of the population at any given time.
Epidemiology
N. meningitidis is found worldwide, but incidence is greatest in the "meningitis belt" of Sub-Saharan Africa (Map 4.12.1). Meningococcal disease is hyperendemic in this region, and periodic epidemics during the dry season (roughly December–June) reach an incidence of up to 1,000 cases per 100,000 population. By contrast, rates of disease in Australia, Europe, South America, and the United States range from 0.1–2.4 cases per 100,000 population per year.
Although meningococcal disease outbreaks can occur anywhere in the world, they are most common in the African meningitis belt, where large-scale epidemics occur every 5–12 years. Historically, outbreaks in the meningitis belt were primarily due to serogroup A. With the introduction of a monovalent serogroup A meningococcal conjugate vaccine (MenAfriVac) in the region starting in 2010, however, recent meningococcal outbreaks in the meningitis belt have primarily been caused by serogroups C and W; serogroup X outbreaks also have been reported.
Outside the meningitis belt, infants, adolescents, and adults >80 years of age have the highest rates of disease. In meningitis belt countries, high rates of disease are seen in people ≤30 years old; the highest rates are in children and adolescents aged 5–14 years.
Unvaccinated travelers visiting meningitis belt countries and having prolonged contact with local populations during an epidemic are at greatest risk for meningococcal disease. The Hajj and Umrah pilgrimages to Saudi Arabia also have been associated with outbreaks of meningococcal disease among returning pilgrims and their contacts, including 6 cases in Umrah participants and their contacts in the United States in 2024 (see Saudi Arabia: Hajj and Umrah Pilgrimages chapter).
Map 4.12.1
Disease data source: World Health Organization (2015). International travel and health. WHO.
Notes
Disease data source: World Health Organization (2015). International travel and health. WHO.
1Meningococcal vaccination is not recommended for travelers to areas outside of the meningitis belt unless there is an ongoing outbreak. See CDC Travelers' Health country pages or travel health notices for up-to-date country-specific meningitis information.
Clinical presentation
Meningococcal disease generally occurs 1–10 days after exposure and presents as meningitis in approximately 50% of cases in the United States. Meningococcal meningitis is characterized by sudden onset of headache, fever, and neck stiffness, sometimes accompanied by nausea, vomiting, photophobia, or altered mental status. Approximately 30% of people with meningococcal disease present with meningococcal sepsis, known as meningococcemia. Symptoms of meningococcemia can include abrupt onset of fever, chills, vomiting, diarrhea, and a petechial or purpuric rash, which can progress to purpura fulminans. Meningococcemia often involves hypotension, acute adrenal hemorrhage, and multiorgan failure. An additional 15% of meningococcal disease cases in the United States, primarily among adults >65 years of age, present as bacteremic pneumonia.
Other presentations (e.g., septic arthritis) also occur. Among infants and children aged <2 years, meningococcal disease can have non-specific symptoms. Neck stiffness, usually seen in people with meningitis, might be absent in this age group.
Meningococcal disease progresses rapidly and has a case-fatality rate of 10%–15%, even with antimicrobial drug treatment. Without rapid treatment, fatality rates can be much higher. In addition, up to 20% of survivors can have long-term health effects such as deafness or amputations due to necrosis of the extremities.
Diagnosis
Early diagnosis and treatment are critical. If bacterial meningitis is suspected, collect blood for culture right away and perform a lumbar puncture (LP) to collect cerebrospinal fluid (CSF) for microscopic examination and Gram stain. In general, diagnosis is made by isolating N. meningitidis from a normally sterile body site (e.g., blood, CSF) either by culture or by PCR detection of N. meningitidis-specific nucleic acid. State health departments can provide diagnostic and testing support if needed.
Signs and symptoms of meningococcal meningitis are like those of other causes of bacterial meningitis (e.g., Haemophilus influenzae, Streptococcus pneumoniae). Proper treatment and prophylaxis depend on correctly identifying the causative organism. Meningococcal disease is nationally notifiable in the United States; report cases to the state or local health department without delay.
Treatment
Meningococcal disease can be rapidly fatal and should always be viewed as a medical emergency. As soon as disease is suspected and blood cultures and CSF have been collected, deliver appropriate treatment; if the LP is to be delayed for any reason (e.g., imaging studies of the head prior to LP), administer antimicrobial drugs immediately after collecting blood cultures. Begin empiric antimicrobial drug treatment early and prior to receiving diagnostic test results.
Third-generation cephalosporins are recommended for empiric treatment. Determine meningococcal isolate susceptibility before switching to penicillin or ampicillin; recent reports indicate emerging penicillin resistance among meningococcal isolates in the United States. If a patient presents with suspected bacterial meningitis of uncertain etiology, some treatment algorithms recommend empiric use of dexamethasone in addition to an antimicrobial drug until a bacterial etiology is established; if meningococcal meningitis is confirmed or suspected, steroids can be discontinued.
Prevention
Vaccine
Five meningococcal vaccines (2 quadrivalent, 2 monovalent, and 1 pentavalent) are licensed and available in the United States. Travelers should receive vaccines 7–10 days before travel to enable time for protective antibody levels to develop. See Table 4.12.1 for more information about available meningococcal vaccines that are recommended for some travelers.
Table 4.12.1: Meningococcal vaccines licensed and available in the United States and recommended for travelers to or residents of countries where meningococcal disease Is hyperendemic or epidemic1
| Vaccine | Trade Name (Manufacturer) | Age at Vaccine Initiation | Dose | Series |
|---|---|---|---|---|
| Meningococcal (serogroups A, C, W, and Y) oligosaccharide diphtheria CRM197 conjugate vaccine (MenACWY-CRM)2 | Menveo (GlaxoSmithKline) | 2 months old | 0.5 mL IM |
4-dose series3 Dose 1: Infant is 2 months old Dose 2: 2 months after dose 1 (infant is 4 months old) Dose 3: 2 months after dose 2 (infant is 6 months old) Dose 4: 6 months after dose 3 (infant is 12 months old) |
| Meningococcal (serogroups A, C, W, and Y) oligosaccharide diphtheria CRM197 conjugate vaccine (MenACWY-CRM)2 | Menveo (GlaxoSmithKline) | 3–6 months old | 0.5 mL IM |
Multidose series3 (number of doses depends on age at vaccine initiation) Dose 1: Infant is between 3–6 months old Subsequent doses: After dose 1, give 1 dose every 8 weeks until the infant is ≥7 months old, then give 1 additional dose after the infant is ≥12 months old |
| Meningococcal (serogroups A, C, W, and Y) oligosaccharide diphtheria CRM197 conjugate vaccine (MenACWY-CRM)2 | Menveo (GlaxoSmithKline) | 7–23 months old | 0.5 mL IM |
2-dose series3 Dose 1: Child is 7–23 months old Dose 2: ≥12 weeks after dose 1 and the child is ≥12 months old |
| Meningococcal (serogroups A, C, W, and Y) oligosaccharide diphtheria CRM197 conjugate vaccine (MenACWY-CRM)2 | Menveo (GlaxoSmithKline) | ≥2 years old | 0.5 mL IM | 1 dose3,4 |
| Meningococcal (serogroups A, C, W, and Y) polysaccharide tetanus toxoid conjugate vaccine (MenACWY-TT) | MenQuadfi (Sanofi Pasteur) | ≥2 years old | 0.5 mL IM | 1 dose3,4 |
| Meningococcal (serogroups A, C, W, and Y) polysaccharide tetanus toxoid conjugate / meningococcal serogroup B vaccine (MenACWY-TT/MenB-FHbp) | Penbraya (Pfizer) | ≥10 years old | 0.5 mL IM | 1 dose; may be used when both MenACWY and MenB vaccines are indicated at the same visit |
Notes
Abbreviations: IM, intramuscular.
1Sources: (1) TABLE 9. Recommended vaccination schedule and intervals for people who travel to or are residents of countries where meningococcal disease is hyperendemic or epidemic—Advisory Committee on Immunization Practices, United States, 2020. (2) Menveo New Ready-to-Use Single Vial Presentation. (3) ACIP Recommendations
2The 1-vial formulation of Menveo can be used in individuals aged ≥10 years; younger persons should receive the 2-vial formulation.
3For people at continued risk, revaccination (booster) with meningococcal conjugate vaccine (MenACWY-CRM, -D, or -TT) is recommended for the following age groups: <7 years old, a single dose 3 years after primary vaccination and every 5 years thereafter; ≥7 years old, a single dose 5 years after primary vaccination and every 5 years thereafter.
4A 2-dose primary series (dose 2 given 8–12 weeks after dose 1) is recommended for the following groups: people with HIV; people with anatomic or functional asplenia; people with persistent complement component deficiency (C3, C5-9, properdin, factor D, factor H); and people taking a complement component inhibitor (e.g., eculizumab [Soliris] or ravulizumab [Ultomiris]).
Routine immunization
The Advisory Committee on Immunization Practices (ACIP) recommends routine administration of a quadrivalent meningococcal conjugate vaccine (MenACWY) for all people aged 11–18 years. Administer a single dose of vaccine to patients at age 11 or 12 years and a booster dose at age 16 years. Routine immunization with MenACWY is not recommended for other age groups in the United States, except for people at increased risk for meningococcal disease, including those with a persistent complement component deficiency (C3, C5-9, properdin, factor D, factor H); people taking a complement component inhibitor (e.g., eculizumab [Soliris] or ravulizumab [Ultomiris]); people who have functional or anatomic asplenia; or people with HIV. ACIP describes vaccine, product, number of doses, and booster dose recommendations, based on age and risk factors for each risk group, in Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020.
ACIP also recommends that adolescents and young adults aged 16–23 years be vaccinated with a serogroup B meningococcal (MenB) vaccine series, based on shared clinical decision-making. A MenB vaccine series provides short-term protection against most strains of serogroup B meningococcus; 16–18 years is the preferred age for MenB vaccination. ACIP also recommends routine use of MenB vaccine for people aged ≥10 years who are at increased risk for meningococcal disease, including people who have persistent complement component deficiency and those with functional or anatomic asplenia. ACIP recommendations for use of MenB vaccines are available online.
A pentavalent meningococcal vaccine including both a MenACWY and a MenB component was licensed in the United States in 2023 and may be used when both MenACWY and MenB vaccines are indicated at the same visit.
Immunization for travelers
Quadrivalent meningococcal conjugate (MenACWY) vaccines
ACIP recommends that travelers aged ≥2 months who visit or reside in parts of the meningitis belt of Sub-Saharan Africa (Map 4.12.1) during the meningitis season (December–June) receive vaccination with a MenACWY vaccine before travel. The Centers for Disease Control and Prevention (CDC) issues advisories for travelers to other countries when outbreaks of meningococcal disease are recognized; travelers should check the CDC Travelers' Health website before travel. There are 2 MenACWY vaccines licensed and available in the United States for children; the age at vaccine initiation and schedule differs for each. See Table 4.12.1 for more information about MenACWY vaccines for young children.
The Kingdom of Saudi Arabia (KSA) requires travelers ≥1 year of age making the Umrah or Hajj pilgrimage to provide documentation of quadrivalent vaccine ≥10 days and ≤3 years before arrival for polysaccharide vaccine (MPSV4, no longer available in the United States) and ≤5 years before arrival for conjugate vaccine. Travelers should confirm visa requirements with the KSA embassy. Pregnant women (see Pregnant Travelers chapter) and children (see Traveling Safely with Infants and Children chapter) should receive meningococcal vaccination according to licensed indications for their age if they travel (see Saudi Arabia: Hajj and Umrah Pilgrimages chapter).
International travelers at risk for meningococcal disease who were previously vaccinated with a quadrivalent vaccine should receive a booster dose. For children who completed the primary dose or series at <7 years of age, administer a booster dose of MenACWY after 3 years and repeat every 5 years thereafter for those who live in or travel to hyperendemic areas. For people who received the primary dose or series at ≥7 years of age, administer a booster dose after 5 years and every 5 years thereafter for people who live in or travel to a hyperendemic area.
Pentavalent meningococcal conjugate (MenACWY-TT/MenB-FHbp) vaccine
A pentavalent serogroup A, B, C, W, Y vaccine is available in the United States and may be used in individuals aged 10 years and older who are indicated to receive both a MenACWY and a MenB vaccine during the same visit. Note that while serogroup B meningococcal vaccines are not specifically recommended for travelers, some travelers may have other indications for receiving serogroup B vaccine as described in the ACIP recommendations. The pentavalent vaccine is not recommended for use if only the MenACWY vaccine is indicated, such as for travelers who have no other indications for meningococcal vaccination.
Other meningococcal vaccines (monovalent serogroups A, B, and C and pentavalent serogroup A, C, W, X, and Y)
Beginning in 2010, the Meningitis Vaccine Project introduced MenAfriVac, a monovalent serogroup A meningococcal conjugate vaccine, into meningitis belt countries through mass vaccination campaigns and the routine childhood immunization schedule. More recently, a serogroup A, C, W, X, Y pentavalent meningococcal vaccine has been developed for use in meningitis belt countries. Neither of these vaccines is licensed for use in the United States. U.S. travelers going to live or work in the meningitis belt should receive a quadrivalent meningococcal conjugate vaccine (MenACWY) before leaving.
MenB vaccine is not routinely recommended for travelers, including for people who live in or travel to meningitis belt countries, because serogroup B disease is extremely rare in this region.
In some countries outside the meningitis belt, meningococcal vaccination (e.g., monovalent conjugate C vaccine or MenB vaccine) might be recommended as part of the routine immunization program for infants. Healthcare professionals can consider meningococcal vaccination for infants residing in these countries, according to the routine immunization recommendations of that country.
Safety and adverse reactions
Side effects after MenACWY vaccination include low-grade fevers and local reactions (e.g., injection-site pain, arm swelling, pain that limits movement of the injected arm). Symptoms are generally mild to moderate and resolve within 48–72 hours. Severe adverse events (e.g., high fever, chills, joint pain, rash, seizures) are rare (<5% of vaccinees).
Although no clinical trials of meningococcal vaccines currently available in the United States have been conducted in women who are pregnant or lactating, post-licensure safety data have not identified any serious safety concerns to the mother or fetus. In addition, limited clinical trial data from use of other meningococcal conjugate vaccines among pregnant women have not identified concerns. Pregnancy or lactation should not preclude vaccination with MenACWY if indicated.
Precautions and contraindications
People with moderate or severe acute illness should defer vaccination until their condition improves. Vaccination is contraindicated for people who have had a severe allergic reaction to any component of the vaccines or to a prior dose of the vaccine. A severe allergic reaction to any diphtheria toxoid- or CRM197-containing vaccine also is a contraindication for MenACWY-CRM; severe allergic reaction to any tetanus toxoid-containing vaccine is a contraindication for MenACWY-TT. All meningococcal vaccines are inactivated and can be given to people who are immunosuppressed.
Post-exposure prophylaxis
In the United States and most industrialized countries, antibiotic chemoprophylaxis is recommended for close contacts of a patient with invasive meningococcal disease to prevent secondary cases. Chemoprophylaxis ideally should be initiated within 24 hours after the index patient is identified; prophylaxis given >2 weeks after exposure has little value.
Antibiotics used for prophylaxis include ceftriaxone, ciprofloxacin, and rifampin. Ceftriaxone is recommended for pregnant women. CDC provides detailed information on meningococcal prophylaxis in the Manual for the Surveillance of Vaccine-Preventable Diseases. Of note, based on detection of multiple ciprofloxacin-resistant cases among travelers, in 2024 CDC recommended preferential consideration for using rifampin, ceftriaxone, or azithromycin instead of ciprofloxacin as prophylaxis for close contacts in the United States of meningococcal disease cases associated with travel to Saudi Arabia.
- Kimberlin, D. W., Banerjee, R., Barnett, E., Lynfield, R., & Sawyer, M. H. (2024). "Meningococcal Infections," Red Book: 2024–2027 Report of the Committee on Infectious Diseases (33rd ed., pp. 585-599). American Academy of Pediatrics. https://publications.aap.org/redbook/book/755/Red-Book-2024-2027-Report-of-the-Committee-on
- Centers for Disease Control and Prevention (CDC). (2001). Update: Assessment of risk for meningococcal disease associated with the Hajj 2001. MMWR: Morbidity and Mortality Weekly Report, 50(12), 221–222. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5012a1.htm
- Chen, M., Guo, Q., Wang, Y., Zou, Y., Wang, G., Zhang, X., Xu, X., Zhao, M., Hu, F., Qu, D., Chen, M., & Wang, M. (2015). Shifts in the Antibiotic Susceptibility, Serogroups, and Clonal Complexes of Neisseria meningitidis in Shanghai, China: A Time Trend Analysis of the Pre-Quinolone and Quinolone Eras. PLoS Medicine, 12(6), e1001838. https://doi.org/10.1371/journal.pmed.1001838
- Collins, J. P., Crowe, S. J., Ortega-Sanchez, I. R., Bahta, L., Campos-Outcalt, D., Loehr, J., Morgan, R. L., Poehling, K. A., & McNamara, L. A. (2024). Use of the Pfizer Pentavalent Meningococcal Vaccine Among Persons Aged ≥10 Years: Recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR. Morbidity and Mortality Weekly Report, 73(15), 345–350. https://doi.org/10.15585/mmwr.mm7315a4
- Epidemic meningitis control in countries of the African meningitis belt, 2016. Lutte contre la méningite épidémique dans les pays de la ceinture africaine de la méningite, 2016. (2017). Releve Epidemiologique Hebdomadaire, 92(13), 145–154.
- Halperin, S. A., Bettinger, J. A., Greenwood, B., Harrison, L. H., Jelfs, J., Ladhani, S. N., McIntyre, P., Ramsay, M. E., & Sáfadi, M. A. (2012). The changing and dynamic epidemiology of meningococcal disease. Vaccine, 30 Suppl 2, B26–B36. https://doi.org/10.1016/j.vaccine.2011.12.032
- Mbaeyi, S. A., Bozio, C. H., Duffy, J., Rubin, L. G., Hariri, S., Stephens, D. S., & MacNeil, J. R. (2020). Meningococcal vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR: Morbidity and Mortality Weekly Report, 69(9), 1–41. https://www.doi.org/10.15585/mmwr.rr6909a1
- McNamara, L. A., Potts, C., Blain, A. E., Retchless, A. C., Reese, N., Swint, S., Lonsway, D., Karlsson, M., Lunquest, K., Sweitzer, J. J., Wang, X., Hariri, S., Fox, L. M., & Antimicrobial-Resistant Neisseria meningitidis Team (2020). Detection of Ciprofloxacin-Resistant, β-Lactamase-Producing Neisseria meningitidis Serogroup Y Isolates - United States, 2019-2020. MMWR. Morbidity and Mortality Weekly Report, 69(24), 735–739. https://doi.org/10.15585/mmwr.mm6924a2
- Tapia, M. D., Sow, S. O., Tamboura, B., Tégueté, I., Pasetti, M. F., Kodio, M., Onwuchekwa, U., Tennant, S. M., Blackwelder, W. C., Coulibaly, F., Traoré, A., Keita, A. M., Haidara, F. C., Diallo, F., Doumbia, M., Sanogo, D., DeMatt, E., Schluterman, N. H., Buchwald, A., Kotloff, K. L., Chen, W. H., Orenstein, E. W., Orenstein, L. A. V., Villanueva, J., Bresee, J., Treanor, J., Levine, M. M. (2016). Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. The Lancet Infectious Diseases, 16(9), 1026–1035. https://doi.org/10.1016/S1473-3099(16)30054-8
- Trotter, C. L., Lingani, C., Fernandez, K., Cooper, L. V., Bita, A., Tevi-Benissan, C., Ronveaux, O., Préziosi, M. P., & Stuart, J. M. (2017). Impact of MenAfriVac in nine countries of the African meningitis belt, 2010-15: an analysis of surveillance data. The Lancet Infectious Diseases, 17(8), 867–872. https://doi.org/10.1016/S1473-3099(17)30301-8
