Purpose
Introduction
Infectious agent
Influenza virus
Endemicity
Worldwide with seasonal variation
Traveler categories at greatest risk for exposure and infection
All travelers
Prevention methods
Influenza is a vaccine-preventable disease
Practice hand hygiene
Avoid touching eyes, nose, or mouth
Avoid close contact with people who are sick
Use appropriate personal protective equipment
Diagnostic support
A clinical laboratory certified in moderate complexity testing; or contact CDC Influenza Laboratory (404-639-2434)
Infectious agent
Influenza is caused by infection of the respiratory tract with influenza viruses, RNA viruses of the Orthomyxovirus genus. Influenza viruses are classified into 4 types: A, B, C, and D. Influenza A and B viruses commonly cause illness in humans and seasonal epidemics. Influenza A viruses are classified into subtypes based on the surface proteins hemagglutinin (HA) and neuraminidase (NA). Two influenza A virus subtypes, A(H1N1) and A(H3N2), and 2 influenza B virus lineages, B-Victoria and B-Yamagata, circulate in humans worldwide; the distribution of these viruses varies year to year and between geographic areas and time of year. Information about circulating seasonal viruses in various regions can be found on the Centers for Disease Control and Prevention (CDC) website or World Health Organization website.
Influenza type C infections generally cause mild illness and are not thought to cause human influenza epidemics. Influenza D viruses primarily affect cattle and are not known to infect or cause illness in people.
Novel influenza A viruses are antigenically different from seasonal influenza viruses and usually circulate among animals. Notably, avian influenza A(H5N1), A(H5N6), A(H7N9) and A(H9N2) viruses and swine-origin variant viruses A(H1N1)v, A(H1N2)v, and A(H3N2)v have resulted in novel human influenza infections globally.
Transmission
Influenza viruses spread from person to person, primarily through respiratory droplets (e.g., when an infected person coughs or sneezes near a susceptible person). Transmission generally occurs via large particle droplets that require close proximity (<2 m; ≤6 ft) between the source and the recipient, but airborne transmission via small particle aerosols can occur within confined air spaces. Indirect transmission occurs when a person touches their face after touching a virus-contaminated surface.
The incubation period is usually 1–4 days after exposure. Most adults ill with influenza shed the virus in the upper respiratory tract and are infectious from the day before symptom onset to approximately 5–7 days after symptom onset. Infectiousness is greatest within 3–4 days after illness onset and is higher in those with fever. Children, immunocompromised people, and severely ill people might shed influenza virus for ≥10 days after symptom onset. Those who are asymptomatic can still shed influenza virus and infect others.
Influenza A virus transmission from animals to humans is rare but possible. Infected birds shed influenza virus in their droppings, mucus, and saliva, and transmission to humans can occur from direct contact with an animal (by touching an infected animal or by droplet spread) or contact with a sick animal's environment (by inhalation of airborne viruses or through fomite transmission). See CDC's Avian Influenza A Virus Infection in Humans website for more details. Infected swine shed influenza virus in nasal secretions and can transmit viruses to humans in the same way seasonal influenza viruses spread among people. For more information, see CDC's website What People Who Raise Pigs Need to Know About Influenza.
Epidemiology
Seasonal influenza
Influenza seasonality varies geographically. The risk for influenza exposure during travel depends on the time of year and destination. In temperate regions, influenza epidemics are more common during cooler months, October–March in the Northern Hemisphere and April–September in the Southern Hemisphere. In subtropical and tropical regions, seasonal influenza epidemics follow a similar pattern, but influenza illnesses can occur throughout the year. During the COVID-19 pandemic in 2020 and 2021, there was a sharp decrease in global influenza activity. Although causality has not been confirmed, the decrease has been attributed, in part, to community and personal implementation of nonpharmaceutical interventions to mitigate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission. These interventions included personal protective measures and community mitigation such as reduced domestic and international travel. Influenza activity has resumed as of 2022. It is unclear how SARS-CoV-2 circulation will impact the circulation of other respiratory viruses, including influenza, in future seasons.
CDC estimates that 9–45 million (symptomatic) illnesses, 4–21 million outpatient visits, 140,000–710,000 hospitalizations, and 12,000–52,000 deaths associated with influenza occur each year in the United States. Globally, annual influenza epidemics result in an estimated 3–5 million cases of severe illness and 290,000–650,000 deaths.
At-risk populations
Certain groups are at increased risk for influenza complications (Box 4.6.1). The incidence of influenza illness is greatest among children, especially those aged 0–4 years. Rates of hospitalization (a marker of severe illness) and death due to influenza are typically higher among older adults (≥65 years old), adults aged 50–64 years, children aged <2 years, and people of any age with underlying medical conditions that place them at increased risk for complications.
Box 4.6.1
Zoonotic influenza
Influenza A viruses circulate among animal populations and occasionally infect humans. The primary reservoirs for influenza A viruses are wild birds, like waterfowl, but influenza A viruses are also common in domestic poultry and swine populations. Influenza A viruses can also infect other animal species (e.g., bats, cats, dairy cattle, dogs, ferrets, horses, sea lions, seals).
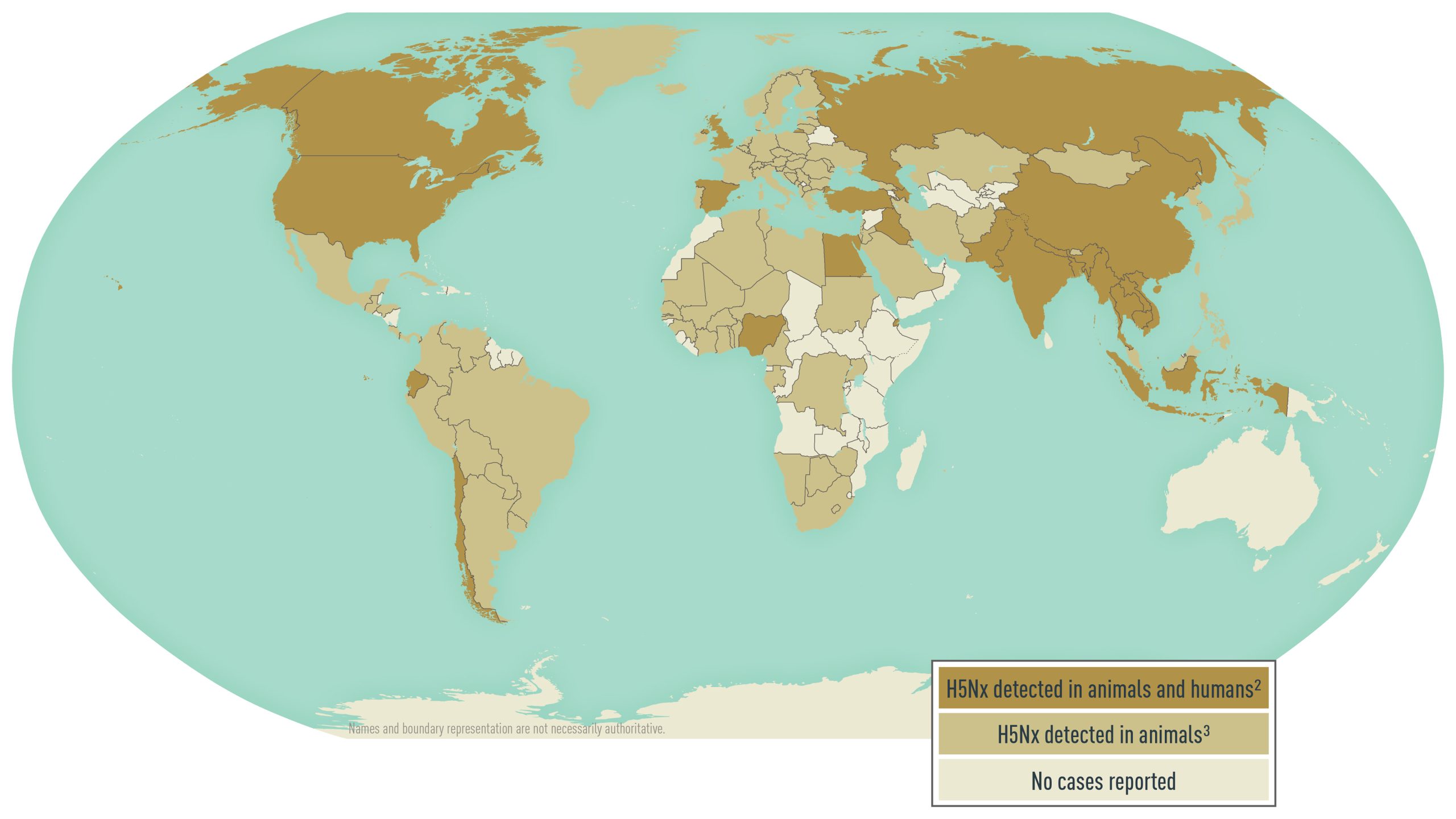
In the United States, the largest outbreak of H5 lineage avian influenza virus in animals started in 2022. Although 34 different avian influenza subtypes have been reported globally since 2005, 96% of outbreaks reported in birds were caused by H5 lineage viruses. Since 2005, more than 35,000 outbreaks of high pathogenicity H5 lineage avian influenza in animals have been reported from 119 countries (Map 4.6.1). Avian influenza virus outbreaks do not have to be reported to the World Organisation for Animal Health (WOAH) if the virus is endemic in a country; avian influenza A(H5N1) was declared endemic in Indonesia in 2006 and in Egypt in 2008. Swine influenza is not reportable to WOAH.
Risk for zoonotic influenza is highest among people with close contact with animals.
Novel influenza A virus human infections
Novel influenza A virus human infections are uncommon but potentially could cause a pandemic if sustained human-to-human transmission occurs. Human infections with influenza viruses that normally circulate in swine (swine influenza viruses) or birds (avian influenza viruses) are not common, but cases are reported globally each year.
Variant influenza viruses
Influenza viruses that normally circulate in pigs (i.e., swine influenza viruses) do not normally infect humans. However, sporadic human infections with influenza viruses that normally circulate in swine have occurred. When this happens, these viruses are called "variant viruses." They are denoted by adding the letter "v" to the end of the virus subtype. Human infections with influenza A(H1N1)v, A(H1N2)v, and A(H3N2)v have been identified in the United States; the largest variant influenza outbreak occurred in 2012 and had a total of 309 infections and 1 death associated with an A(H3N2)v virus.
From 2013 through 2023, 165 human infections with variant influenza viruses were identified in 27 U.S. states. Most people identified with variant virus infections reported contact with swine preceding their illness, suggesting swine-to-human transmission. Limited cases of human-to-human transmission of variant viruses have also been reported. Seasonal human influenza viruses have infected swine, suggesting person-to-swine transmission. Agricultural fairs and swine farms are settings in which humans are exposed to swine. Illnesses associated with variant influenza virus infections are usually mild, with symptoms similar to those of seasonal influenza. However, there is a risk that co-circulation of seasonal and variant influenza viruses in susceptible persons (or pigs) could generate a reassorted virus, which could lead to a pandemic if the reassorted virus is capable of efficient human-to-human transmission.
Avian influenza A(H5) lineage viruses
During 2013–2023, 16 countries reported over 330 human infections with avian influenza A(H5) lineage viruses, with a reported case-fatality ratio of approximately 34% (Map 4.6.1). Cases ranged in age from 0 to 81 years, with an average age of 27 years. Most disease from A(H5) lineage viruses occurred after direct or close contact with sick or dead infected poultry or their environments. Influenza A(H5N1) and A(H5N6) viruses are widespread among poultry in some countries in Asia and the Middle East; China accounts for 99% of A(H5N6) infections in humans globally. Starting in 2022, A(H5N1) spread through wild bird migration, with countries on 5 continents reporting detections of A(H5N1) in wild birds or poultry. Rare instances of limited human-to-human A(H5N1) virus transmission have been reported. Since 2022, the United States has reported a limited number of cases of human infection with A(H5N1), most with known exposure to infected animals.
Map 4.6.1
Notes
1Disease data source: Animal disease events. World Organisation for Animal Health, World Animal Health Information System (WOAH-WAHIS).
2H5Nx lineages include: H5N1, H5N6, and H5N8.
3H5Nx lineages include: H5N1, H5N2, H5N3, H5N4, H5N5, H5N6, H5N8, H5N9, and H5Nx.
Avian influenza A(H7N9) virus
Avian influenza A(H7N9) virus emerged in China in 2013. Since then, it has caused 1,568 confirmed human infections, but there have been no new human cases detected since 2019. Most cases have been identified in mainland China, but several infections have been identified in Hong Kong Special Administrative Region (SAR), Macau SAR, Malaysia, and Taiwan, in travelers who reported exposure in mainland China. In 2014, Canada reported the first human influenza A(H7N9) virus infection in North America in a traveler returning from China. Most people (70%) with A(H7N9) virus infection had a known exposure to infected poultry or contaminated environments (e.g., live bird markets). The virus has been found in poultry and environmental specimens collected in China. Most reported human A(H7N9) infections have resulted in severe respiratory illnesses; the reported case-fatality ratio is 40%.
Other avian influenza viruses
Although uncommon, human infections with other avian influenza viruses, including A(H3N8), A(H7N2), A(H7N3), and A(H10N3), have been reported globally in recent years, including 2 cases of A(H7N2) in humans exposed to infected cats in New York in 2016. Even though human infections with avian influenza viruses in the United States are limited, surveillance in birds, cattle, and people exposed to infected animals is ongoing because of the low, but continued, risk for transmission to humans and the chance of reassortment with human-to-human transmission capability emerging, resulting in pandemic.
Clinical presentation
Physical findings
Influenza illness is characterized by an abrupt onset of signs and symptoms that include rhinitis, nonproductive cough, sore throat, headache, fever, chills, malaise, shortness of breath, earache, muscle aches, vomiting, or, less commonly, rash. Illness without fever can occur, especially in older adults and infants. Children are more likely than adults to experience nausea, vomiting, or diarrhea when ill with influenza.
Physical findings are predominantly localized to the respiratory tract and include nasal discharge, pharyngeal inflammation without exudates, and occasionally rales on chest auscultation. Uncomplicated influenza illness typically resolves within 1 week for most previously healthy children and adults who do not receive antiviral medication, although cough and malaise can persist for >2 weeks, especially in older adults.
Humans infected with variant influenza viruses have a clinical presentation like seasonal influenza virus infections. Reported human infections with avian influenza A(H5N1) or A(H7N9) viruses can present with conjunctivitis, upper respiratory infection, or severe pneumonia. Severity data might be skewed, however, because people with less severe illness often do not seek care for influenza or get tested for avian-origin A(H5) or A(H7) viruses.
Complications
Complications of influenza virus infection include primary influenza viral pneumonia and secondary bacterial pneumonia; also, co-infections with other viral or bacterial pathogens, encephalopathy, exacerbation of underlying medical conditions (e.g., cardiac disease, pulmonary disease), Guillain-Barré syndrome, myocarditis, myositis, parotitis, seizures, and rarely, death.
Diagnosis
Influenza cannot be distinguished from other respiratory illnesses caused by other pathogens based on signs and symptoms alone. The positive predictive value of clinical signs and symptoms for influenza-like illness (fever with either cough or sore throat) for laboratory-confirmed influenza virus infection is 30%–88%, depending on host factors (e.g., age, community influenza activity levels).
Diagnostic testing
Consider diagnostic testing for: hospitalized patients with suspected influenza; all patients for whom a diagnosis of influenza will inform clinical care decisions, including patients who do not improve on antiviral therapy and those with medical conditions that place them at increased risk for complications; and patients for whom results of influenza testing would affect infection control or management of close contacts, including other patients, such as in institutional outbreaks or other settings (e.g., cruise ships, tour groups).
For healthcare professionals seeking laboratory confirmation of influenza, the Infectious Diseases Society of America recommends the use of rapid molecular assays in outpatients and nucleic acid amplification tests (e.g., reverse transcription PCR [RT-PCR]) in hospitalized patients. For suspected human infection with a novel influenza A virus of animal origin (e.g., avian influenza A virus, swine influenza A virus), contact the local and state health departments to perform RT-PCR for seasonal influenza viruses and novel influenza A viruses. Other diagnostic tests available for influenza include antigen-based rapid influenza diagnostic tests, multiplex assays that detect both influenza and SARS-CoV-2, immunofluorescence assays, and viral culture. Most patients with clinical illness consistent with uncomplicated influenza in communities where influenza viruses are circulating do not require diagnostic testing for empiric clinical management. Healthcare professionals should not wait for the results to initiate empiric antiviral treatment for influenza in priority groups, including hospitalized patients with respiratory illness; outpatients with severe, complicated, or progressive respiratory illness; and outpatients at higher risk for influenza complications who present with any acute respiratory illness symptoms (with or without fever). Co-infection with influenza virus and SARS-CoV-2 is possible and should be considered, particularly in hospitalized patients with severe respiratory disease. Use of multiplex assays that detect both influenza and SARS-CoV-2 can inform clinical management.
Test sensitivity
Nucleic acid assays are the most sensitive diagnostic assays. Thus, if infection with zoonotic or novel influenza viruses is suspected, contact the state health department in the United States or CDC outside the United States. Do not delay starting antiviral treatment while waiting for confirmatory laboratory testing results.
The sensitivity of antigen-based rapid influenza diagnostic tests varies but is substantially lower than RT-PCR or viral culture. Antigen-based rapid influenza diagnostic tests cannot distinguish between seasonal influenza A virus infections and animal-origin influenza A virus infections, and their sensitivity to detect these animal-origin influenza viruses can vary by test type and virus subtype. Therefore, a negative antigen-based rapid influenza diagnostic test result does not rule out influenza virus infection, and healthcare professionals should not rely on a negative antigen-based rapid influenza diagnostic test result to make decisions about treatment.
Treatment
Antiviral treatment
Early antiviral treatment (Table 4.6.1) can shorten the duration of fever and other symptoms and reduce the risk for complications from influenza. Antiviral treatment is recommended as early as possible for any patient with confirmed or suspected influenza who is hospitalized; who has severe, complicated, or progressive illness; or who is at increased risk for influenza-associated complications.
Treatment is most effective if it can be initiated ≤48 hours of symptom onset. For hospitalized patients, those with severe illness, or those at higher risk for complications, antiviral therapy might still be beneficial if started >48 hours after illness onset. Four antiviral agents approved by the U.S. Food and Drug Administration (FDA) for the treatment and prophylaxis of influenza are available: oral oseltamivir, available as a generic (or as Tamiflu, Genentech); intravenous peramivir (Rapivab, BioCryst Pharmaceuticals); inhaled zanamivir (Relenza, GlaxoSmithKline); and oral baloxavir (Xofluza, Genentech).
Oseltamivir is the recommended treatment for people of all ages and is the preferred agent to treat patients who can tolerate oral medications who are hospitalized, have severe or complicated influenza illness, or who are at higher risk of influenza complications. Peramivir is approved and recommended to treat patients aged ≥2 years and might be useful in patients unable to tolerate or absorb oral antiviral therapy. Zanamivir is approved and recommended to treat patients aged ≥7 years and for prophylaxis in people aged ≥5 years. Inhaled zanamivir is not recommended for use in people with underlying chronic respiratory disease. Baloxavir is indicated to treat acute uncomplicated influenza in otherwise healthy patients ≥5 years of age and patients with high-risk conditions ≥12 years of age who have been symptomatic for ≤48 hours.
Two other FDA-approved influenza antiviral medications, amantadine and rimantadine, are not recommended for treatment or prophylaxis of influenza because of widespread viral resistance. Discuss antiviral treatment options with people at increased risk for complications of influenza before they travel to areas with influenza activity.
Table 4.6.1: Treatment and prophylaxis for influenza A and B, approved and recommended antiviral medication dosing schedules
| Antiviral | Route | Use | Pediatric Dose | Adult Dose |
|---|---|---|---|---|
| Oseltamivir | Oral (PO) | Treatment1 |
<1 year old: 3 mg/kg PO, days2 ≥1 year old (weight-based dosing schedule): |
75 mg PO 2×/day ×5 days |
| Prophylaxis1 |
<3 months old: unless the situation is judged critical, oseltamivir is not recommended due to limited data in this age group ≥3 months and <1-year old: 3 mg/kg/dose PO 1×/day ×7 days2 ≥1 year old (weight-based dosing schedule): |
75 mg PO 1×/day ×7 days | ||
| Peramivir | Intravenous (IV) | Treatment3 | 2–12 years old: 12 mg/kg dose (up to 600 mg maximum) by IV infusion over ≥15 minutes ×1 | ≥13 years old: 600 mg by IV infusion over ≥15 minutes ×1 |
| Prophylaxis4 | Not recommended | Not approved | ||
| Zanamivir | Inhaled | Treatment5 | ≥7 years old: 10 mg (2 5-mg inhalations) 2×/day ×5 days | |
| Prophylaxis | ≥5 years old: 10 mg (2 5-mg inhalations) 1×/day ×7 days | |||
| Baloxavir | Oral (PO) | Treatment6 | ≥5 year old (weight-based dosing schedule): 40 to <80 kg: 40 mg PO ×1 ≥80 kg: 80 mg PO ×1 |
|
| Post-exposure prophylaxis | ≥5 year old (weight-based dosing schedule): 40 to <80 kg: 40 mg PO ×1 ≥80 kg: 80 mg PO ×1 |
|||
Notes
1Oseltamivir is approved by the U.S. Food and Drug Administration (FDA) for the treatment of acute uncomplicated influenza ≤48 hours of illness onset. Although not part of the FDA-approved indications, use of oseltamivir to treat influenza in infants <14 days old, and for prophylaxis in infants 3 months to 1 year of age, is recommended by the Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics (AAP).
2AAP provides alternative dosing guidelines for infants aged 9–11 months and for premature infants.
3Peramivir is FDA approved and recommended for treatment of acute uncomplicated influenza ≤48 hours of illness onset. Daily dosing for a minimum of 5 days was used in clinical trials of hospitalized patients with influenza.
4No data for use of peramivir for influenza chemoprophylaxis are available.
5Zanamivir is FDA approved and recommended for treatment of acute uncomplicated influenza ≤48 hours of illness onset.
6Baloxavir marboxil is FDA approved and recommended for treatment of acute uncomplicated influenza ≤48 hours of illness onset.
Prevention
Vaccines
Vaccination is the most effective way to prevent influenza and its complications. In the United States, CDC recommends annual seasonal influenza vaccination for people aged ≥6 months who do not have contraindications. Several influenza vaccines are approved for use in the United States and can be grouped into 3 categories: inactivated influenza vaccines (IIVs, including cell-based, egg-based, high-dose, and adjuvanted influenza vaccines); live attenuated influenza vaccine (LAIV); and recombinant influenza vaccine (RIV).
See updates and recommendations. All recipients should receive an age-appropriate vaccine (one that is approved for their age). Children aged 6 months to 8 years who have never received an influenza vaccine, who have not previously received a lifetime total of ≥2 doses, or whose influenza vaccination history is unknown require 2 doses of age-appropriate influenza vaccine given ≥4 weeks apart to induce sufficient immune response.
Travelers—including people at increased risk for complications of influenza—who did not receive the current seasonal influenza vaccine and who are traveling to parts of the world where influenza activity is ongoing, should consider influenza vaccination ≥2 weeks before departure.
Administration
IIVs and RIV are administered by intramuscular injection. There are IIVs that are licensed for people as young as 6 months of age, but specific age indications and dose volumes vary by product; follow label instructions. In the United States, standard-dose unadjuvanted IIVs (which include egg-based and cell-based inactivated vaccines) are approved for people aged ≥6 months. High-dose and adjuvanted IIVs, which can elicit higher levels of antibodies than standard-dose vaccines, are approved for people aged ≥65 years. RIV is approved for use in people aged ≥18 years.
LAIV is administered as a nasal spray and is labeled for use in people aged 2–49 years in the United States. LAIV is not recommended for women who are pregnant or who have certain medical conditions (see Contraindications and Precautions section below). There are no preferential recommendations for any vaccine type or brand for people aged 6 months through 64 years. People aged ≥65 years should preferentially receive high-dose IIV, RIV, or adjuvanted IIV. If none of these 3 vaccines are available, then any other age-appropriate influenza vaccine should be used.
In general, IIVs, RIV, and LAIV can be administered simultaneously with other inactivated or live vaccines. If LAIV is not administered at the same time as another needed live vaccine, LAIV and the other live vaccine need to be administered ≥4 weeks apart.
Adverse reactions
Inactivated influenza vaccine
The most frequent side effects of vaccination with IIV in adults are soreness and redness at the vaccination site. These local injection-site reactions are slightly more common with high-dose and adjuvanted IIVs. Reactions generally are mild and rarely interfere with the ability to conduct usual, daily activities. Fever, headache, malaise, myalgia, and other systemic symptoms sometimes occur after vaccination; symptoms might be more frequent in people with no previous exposure to the influenza virus antigens in the vaccine (e.g., young children) and are generally short-lived.
Guillain-Barré syndrome is associated with influenza-like illness and was associated with the 1976 swine influenza vaccine, which had an increased risk of 1 additional case of Guillain-Barré syndrome per 100,000 people vaccinated. None of the studies of influenza vaccines other than the 1976 influenza vaccine have demonstrated a risk for Guillain-Barré syndrome of similar magnitude. The increased risk for Guillain-Barré syndrome after seasonal influenza vaccines generally is small, approximately 1–2 additional cases per 1 million people vaccinated, whereas the estimated risk for Guillain-Barré syndrome after influenza has been estimated to be approximately 17.2 cases per 1 million influenza-coded healthcare visits in 1 study.
Live attenuated influenza vaccine
The most frequent side effects of LAIV reported in healthy adults include minor upper respiratory symptoms, runny nose, and sore throat, which are generally well-tolerated. Some children and adolescents have reported fever, myalgia, vomiting, or wheezing. These symptoms, particularly fever, are more often associated with the first administered LAIV dose and are self-limited.
Children aged 2–4 years who have a history of wheezing in the past year or who have a diagnosis of asthma should not receive LAIV. People 2–49 years of age who have conditions that increase their risk for severe influenza (e.g., immunocompromising conditions, pregnancy) should receive IIV or RIV, not LAIV. To decrease the risk of transmitting live virus to severely immunocompromised people (those who require a protected environment), their caretakers also should not receive LAIV or should avoid contact for 7 days after receiving LAIV (for other conditions in which LAIV should not be used, see Contraindications and Precautions section later in the chapter).
Composition
Influenza vaccine composition can be trivalent, protecting against 3 different influenza viruses (2 influenza A subtypes and 1 influenza type B lineage), or quadrivalent, with protection against 4 different influenza viruses (2 influenza A subtypes and 2 influenza type B lineages). Quadrivalent vaccine includes representative strains from antigenically distinct B-Victoria and B-Yamagata lineages. For the 2024–2025 influenza season, all influenza vaccines available in the United States are trivalent vaccines due to a current absence of detection of naturally occurring B/Yamagata.
Coverage
There is no recommendation for revaccination of persons vaccinated during the October–May Northern Hemisphere influenza season who subsequently travel to a Southern Hemisphere country during the April–September Southern Hemisphere influenza season. Southern Hemisphere formulation influenza vaccines might differ in antigen composition from Northern Hemisphere vaccines and might offer protection against different viruses; however, Southern Hemisphere influenza vaccine formulations are not generally commercially available in the United States. People at increased risk for influenza complications should consult with their healthcare professional to discuss the risk for influenza or other travel-related diseases before traveling.
Seasonal influenza vaccines are not expected to provide protection against human infection with animal-origin novel influenza A viruses, including influenza A(H5N1) and A(H7N9) viruses. While there may be stockpiles of animal-origin influenza virus vaccines for use during emergencies, no commercially available influenza vaccines are available to protect against avian or variant influenza viruses.
Contraindications and precautions
Contraindications
Each individual influenza vaccine is contraindicated in people who have had a severe allergic reaction to any component of that vaccine, except egg. Although package inserts for U.S. approved, egg-based influenza vaccines list previous severe allergic reaction to egg as a contraindication to these vaccines, the Advisory Committee on Immunization Practices (ACIP) recommends that people with a history of egg allergy of any severity can receive any licensed and recommended influenza vaccine (egg-based or non-egg-based) that is otherwise appropriate for their age and health status. Vaccine components can be found in package inserts.
In addition to allergies to vaccine components, egg-based IIVs and LAIV are contraindicated in people who have had a previous severe allergic reaction to any influenza vaccine. Cell-based IIV is contraindicated for people who have had a previous severe allergic reaction to any cell-based IIV; RIV is contraindicated for people who have had a severe allergic reaction to any RIV (for more information on use of cell-based IIV and RIV for people who have had a severe allergic reaction to an influenza vaccine, see Precautions section below).
In addition to the contraindications listed above, LAIV is contraindicated in children and adolescents who are taking aspirin- and salicylate-containing medications. LAIV should also not be administered to women who are pregnant, people who are immunocompromised, people who are close contacts or caregivers of people who are severely immunocompromised and require a protected environment, who have a cerebrospinal fluid leak, who have cochlear implants, or to children aged 2 through 4 years with a history of asthma or wheezing. Recent receipt of influenza antiviral medications might interfere with the effectiveness of LAIV; consult the current ACIP recommendations for further details.
Precautions
Prior Guillain-Barré syndrome within 6 weeks after receipt of any influenza vaccine is a precaution to the use of all influenza vaccines. Moderate or severe acute illness with or without fever is a precaution for all vaccines. Severe allergic reaction to any egg-based IIV, RIV, or LAIV is a precaution to cell culture IIV. Severe allergic reaction to any egg-based IIV, ccIIV, or LAIV is a precaution to RIV. For LAIV, precautions include asthma in persons aged ≥5 years and other underlying medical conditions that predispose to severe influenza illness (e.g., chronic pulmonary, cardiovascular [except isolated hypertension], renal, hepatic, neurologic, hematologic, or metabolic disorders [including diabetes mellitus]).
Post-exposure prophylaxis
Influenza antiviral drugs can be used for prophylaxis to prevent infection after close contact with a confirmed case. CDC does not, however, recommend routine use of antiviral medications for prophylaxis except as one of multiple interventions to control institutional influenza outbreaks. Initiate post-exposure prophylaxis ≤48 hours of exposure but never >48 hours because of the risk of treating infection with a subtherapeutic dose. Alternatively, exposed people can monitor for symptoms and initiate antiviral treatment early after symptoms begin.
CDC recommendations for antiviral use for variant influenza virus infections are like those for seasonal influenza virus infection. CDC recommends antiviral treatment for all suspected cases of human infection with avian influenza viruses. See recommendations for post-exposure prophylaxis of close contacts of confirmed human infections of avian influenza viruses. Post-exposure prophylaxis is not routinely recommended for those exposed to birds infected with influenza. However, prophylaxis may be considered based on clinical judgement. If antiviral prophylaxis is initiated for people exposed to avian influenza A viruses, CDC recommends twice daily dosing for oseltamivir or zanamivir instead of once daily dosing.
Personal protective measures
Measures that can help prevent influenza virus infection and other infections during travel include avoiding close contact with sick people and washing hands often with soap and water. In places where soap and a safe source of water are not available, CDC recommends using an alcohol-based hand sanitizer containing ≥60% alcohol. Face coverings are effective in preventing the spread of some respiratory viruses, particularly among people in confined areas, and might have a role in the prevention of contagion during influenza epidemic periods. An ill person can help prevent the spread of illness to others by covering their nose and mouth with their elbow when coughing and sneezing and avoiding close contact with others. If symptomatic people cannot avoid contact with others, consider having them wear a mask when they are in close contact with others.
The best way to prevent infection with animal-origin influenza viruses, including A(H5N1) and variant influenza viruses, is to follow standard travel safety precautions, including using good hand hygiene, practicing food safety precautions, and avoiding contact with sources of exposure. Most human infections with animal-origin influenza viruses have occurred after direct or close contact with infected poultry or swine or their contaminated environments. In destinations where avian influenza virus outbreaks are occurring, travelers or those living abroad should avoid live animal markets and farms where animals are raised, avoid contact with sick or dead animals, avoid eating undercooked or raw animal products (including eggs), and avoid eating foods or drinking beverages that contain animal blood. For those who cannot avoid live animal markets and farms, the use of respiratory protection is recommended at a minimum. If working with livestock, additional personal protective equipment (such as googles, coveralls, boots, gloves, etc.) may be warranted.
- Committee on Infectious Diseases. (2022). Recommendations for prevention and control of influenza in children, 2022–2023. Pediatrics, 150(4), 1–10. https://www.doi.org/10.1542/peds.2022-059274
- Groskopf, L. A., Blanton, L. H., Ferdinands, J. M., Chung, J. R., Broder, K. R., & Talbot, H. K. (2023). Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 influenza season. MMWR: Morbidity and Mortality Weekly Report, 72(RR-2), 1–25. doi:https://dx.doi.org/10.15585/mmwr.rr7202a1
- Hirve, S., Newman, L. P., Paget, J., Azziz-Baumgartner, E., Fitzner, J., Bhat, N., Vandemaele, K., Zhang, W. (2016). Influenza seasonality in the tropics and subtropics: When to vaccinate? PloS One, 11(4), e0153003. https://www.doi.org/10.1371/journal.pone.0153003
- Kakoullis, L., Steffen, R., Osterhaus, A., Goeijenbier, M., Rao, S. R., Koiso, S., Hyle, E. P., Ryan, E. T., LaRocque, R. C., Chen, L. H. (2023). Influenza: Seasonality and travel-related considerations. Journal of Travel Medicine, 30(5), 1–12. https://www.doi.org/10.1093/jtm/taad102
- Merckx, J., Wali, R., Schiller, I., Caya, C., Gore, G. C., Chartrand, C., Dendukuri, N., Papenburg, J. (2017). Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: A systematic review and meta-analysis. Annals of Internal Medicine, 167(6), 394–409. https://www.doi.org/10.7326/M17-0848
- Rolfes, M. A., Foppa, I. M., Garg, S., Flannery, B., Brammer, L., Singleton, J. A., Burns, E., Jernigan, D., Olsen, S., Bresee, J., Reed, C. (2018). Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza and Other Respiratory Viruses, 12(1), 132–137. https://www.doi.org/10.1111/irv.12486
- Szablewski, C. M., Iwamoto, C., Olsen, S. J., Greene, C. M., Duca, L. M., Davis, C. T., Davis, C. T., Coggeshall, K. C., Davis, W. W., Emukule, G. O., Gould, P. L., Fry, A. M., Wentworth, D. E., Dugan, V. G., Kile, J. C., Azziz-Baumgarter, E. (2023). Reported global avian influenza detections among humans and animals during 2013–2022: Comprehensive review and analysis of available surveillance data. JMIR Public Health and Surveillance, 9, 1–14. https://www.doi.org/10.2196/46383
- Tsang, T. K., Lau, L. L. H., Cauchemez, S., & Cowling, B. J. (2016). Household transmission of influenza virus. Trends in Microbiology, 24(2), 123–133. https://www.doi.org/10.1016/j.tim.2015.10.012
- Uyeki, T. M., Bernstein, H. H., Bradley, J. S., Englund, J. A., File, T. M., Fry, A. M., Gravenstein, S., Hayden, F. G., Harper, S. A., Hirshon, J. M., Ison, M. G., Johnston, B. L., Knight, S. L., McGeer, A., Riley, L. E., Wolfe, C. R., Alexander, P. E., Pavia, A. T. (2019). Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clinical Infectious Diseases, 68(6), 895–902. https://www.doi.org/10.1093/cid/ciy874
- Uyeki, T. M., Hui, D. S., Zambon, M., Wentworth, D. E., & Monto, A. S. (2022). Influenza. The Lancet, 400(10353), 693–706. https://www.doi.org/10.1016/S0140-6736(22)00982-5
