Purpose

Introduction
Popular high-altitude travel destinations include Colorado ski resorts such as Vail and Breckenridge, with lodgings at 2,480 and 2,925 m (8,150 and 9,600 ft), respectively; Cusco, Peru (approximately 3,350 m; 11,000 ft); La Paz, Bolivia (approximately 3,650 m; 12,000 ft); Lhasa, Tibet Autonomous Region (approximately 3,700 m; 12,100 ft); Everest base camp, Nepal (approximately 5,400 m; 17,700 ft); and Mount Kilimanjaro, Tanzania (approximately 5,900 m; 19,341 ft). High-altitude environments expose travelers to cold, low humidity, increased ultraviolet radiation, and decreased air pressure, all of which can cause health problems. The biggest concern, however, is hypoxia, due to the decreased partial pressure of oxygen (PO2). At around 3,050 m (approximately 10,000 ft), for example, the inspired PO2 is only 69% of that at sea level; acute exposure to this reduced PO2 can lower arterial oxygen saturation to 88%–91%.
The magnitude and consequences of hypoxic stress depend on the altitude, rate of ascent, and duration of exposure; host genetic factors may also contribute. Hypoxemia is greatest during sleep; day trips to high-altitude destinations with an evening return to a lower altitude are much less stressful on the body. Because of the key role of increased ventilation on ascent to high altitudes, travelers with compromised lung function must be cautious, and all travelers should avoid taking respiratory depressants.
Acclimatization
The human body can adjust to moderate hypoxia at altitudes up to approximately 5,200 m (≤17,000 ft) but requires time to do so. Some acclimatization to high altitude continues for weeks to months, but the acute process, which occurs over the first 3–5 days following ascent, is crucial for travelers. The acute phase is associated with a steady increase in ventilation, improved oxygenation, and changes in cerebral blood flow. Increased red cell production does not play a role in acute acclimatization, although a decrease in plasma volume over the first few days does increase hemoglobin concentration.
Altitude illness can develop before the acute acclimatization process is complete, but not afterward. In addition to preventing altitude illness, acclimatization improves sleep, increases comfort and sense of well-being, and improves submaximal endurance; maximal exercise performance at high altitude will always be reduced compared to that at low altitude.
Travelers can optimize acclimatization by adjusting their itineraries to avoid going "too high too fast" (Box 3.5.1). Gradually ascending to altitude or staging the ascent provides crucial time for the body to adjust. For example, acclimatizing for a minimum of 2–3 nights at around 2,450 to approximately 2,750 m (8,000–9,000 ft) before proceeding to a higher altitude is markedly protective against acute mountain sickness (AMS). The Wilderness Medical Society recommends avoiding ascent to a sleeping altitude of 2,750 m (≥9,000 ft) in a single day; ascending at a rate of no greater than 500 m (1,650 ft) per night in sleeping altitude once above 3,000 m (9,800 ft); and allowing an extra night to acclimatize for every 1,000 m (3,300 ft) of sleeping altitude gain. These reasonable recommendations can still be too fast for some travelers and too slow for others.
Box 3.5.1
Altitude illness
Risk to travelers
Susceptibility and resistance to altitude illness are, in part, genetically determined traits, but there are no simple screening tests to predict risk. Training and physical fitness do not affect risk. A traveler's sex plays a minimal role, if any, in determining predisposition. Children are as susceptible as adults; people aged >50 years have slightly less risk. Any unacclimatized traveler proceeding to a sleeping altitude of ≥2,450 m (≥8,000 ft)—and sometimes lower—is at risk for altitude illness. In addition, travelers who have successfully adjusted to an altitude are at risk when moving to higher sleeping altitudes, especially if the altitude gain is 600–900 m (>2,000–3,000 ft).
How a traveler previously responded to high altitude is the most reliable guide for future trips but only if the altitude and rate of ascent are similar, and even then, this is not an infallible predictor. In addition to inherent susceptibilities, a traveler's risk for developing altitude illness is influenced by 2 other main factors: altitude of entry to high altitude and the subsequent rate of ascent (Table 3.5.1). Creating an itinerary to avoid any occurrence of altitude illness is difficult because of variations in individual susceptibility as well as in starting points and terrain. The goal for the traveler might not be to avoid all symptoms of altitude illness but to have no more than mild illness, thereby avoiding itinerary changes or the need for medical assistance or evacuation.
Table 3.5.1: Risk categories for developing acute mountain sickness (AMS)*
| Variable | Low | Medium | High |
|---|---|---|---|
| History of acute altitude illness | None or mild AMS | Moderate to severe AMS | HAPE or HACE |
| Sleeping altitude on day 1 | 2,750 m (<9,000 ft) |
2,750–3,400 m (9,000–11,150 ft)
|
>3,400 m (>11,150 ft) |
| Ascent rate (change in sleeping altitude) | 500 m (<1,650 ft)/day above 3,000 m (9,800 ft) | 500 m (>1,650 ft)/day above 3,000 m (9,800 ft) with extra days for acclimatization every 1,000 m (3,300 ft) | 500 m (>1,650 ft)/day above 3,000 m (9,800 ft) without extra days for acclimatization every 1,000 m (3,300 ft) |
Notes
*Assumes starting altitude <1,200 m (<4,000 ft)
Abbreviations: HACE, high-altitude cerebral edema; HAPE, high-altitude pulmonary edema; AMS, acute mountain sickness.
Recommendations for each category in Table 3.5.1 are as follows:
- Low risk: Acetazolamide prophylaxis is not indicated. The traveler should carry over-the-counter analgesics for headache and have acetazolamide to speed acclimatization as necessary or to treat early AMS.
- Medium risk: Acetazolamide prophylaxis would be beneficial; consider its use. Have the traveler carry acetazolamide for prevention or treatment of AMS and consider prescribing dexamethasone for emergency use.
- High risk: Strongly encourage acetazolamide prophylaxis, and have the traveler carry dexamethasone for emergency use.
Destinations with risk
Some common high-altitude destinations require rapid ascent by airplane to >11,150 ft (>3,400 m), placing travelers in a high-risk category for AMS. A common travel medicine question is whether to recommend acetazolamide for travelers when gradual or staged acclimatization is not feasible. With rates of altitude illness approaching 50% in these situations, a low threshold for chemoprophylaxis is advised. In some cases (e.g., Cusco and La Paz), travelers can descend to elevations lower than the airport to sleep for 1–2 nights and then begin their ascent, perhaps obviating the need for medication.
Itineraries along some trekking routes in Nepal, particularly Everest base camp, push the limits of many people's ability to acclimatize. Even on standard acclimatization schedules, the prevalence of altitude illness can approach 30% at higher elevations. Whenever possible, adding extra days to the trek can make for a more enjoyable and safer trip.
Altitude illness syndromes
Altitude illness is divided into 3 syndromes: AMS; high-altitude cerebral edema (HACE); and high-altitude pulmonary edema (HAPE). Some healthcare professionals consider high-altitude headache a separate entity because isolated headache can occur without the combined symptoms that define AMS.
Acute mountain sickness
AMS is the most common form of altitude illness, affecting, for example, 25% of all visitors sleeping at altitudes >2,450 m (>8,000 ft) in Colorado.
Diagnosis
Diagnosis of AMS is based on a history of recent ascent to high altitude and the presence of subjective symptoms. AMS symptoms are like those of an alcohol hangover; headache is the cardinal symptom, usually accompanied by ≥1 of the following: anorexia, dizziness, fatigue, nausea, or, occasionally, vomiting. Uncommonly, AMS presents without headache. Symptom onset is usually 2–12 hours after initial arrival at a high altitude or after ascent to a higher elevation and often during or after the first night. Preverbal children with AMS can develop loss of appetite, irritability, and pallor. AMS generally resolves within 12–48 hours if travelers do not ascend farther.
The condition is typically self-limited, developing and resolving over 1–3 days. Symptoms starting after 3 days of arrival at high altitude and without further ascent should not be attributed to AMS. AMS has no characteristic physical findings; pulse oximetry is usually within the normal range for the altitude or slightly lower than normal, while a high arterial oxygen saturation (SpO2) for the altitude seems to be protective of AMS. With pulse oximeters available for under $30, travelers may want to have a pulse oximeter with them to gauge their acclimatization progress. Figure 3.5.1 shows the expected range of SpO2 for a given altitude.
The differential diagnosis of AMS is broad; common considerations include alcohol hangover, carbon monoxide poisoning, dehydration, drug intoxication, exhaustion, hyponatremia, and migraine. Unlike viral syndromes, there is no coryza, fever, chills, or myalgia. Travelers with AMS will improve rapidly with descent ≥300 m (≥1,000 ft), and this can be a useful indication for a diagnosis of AMS.
Figure 3.5.1
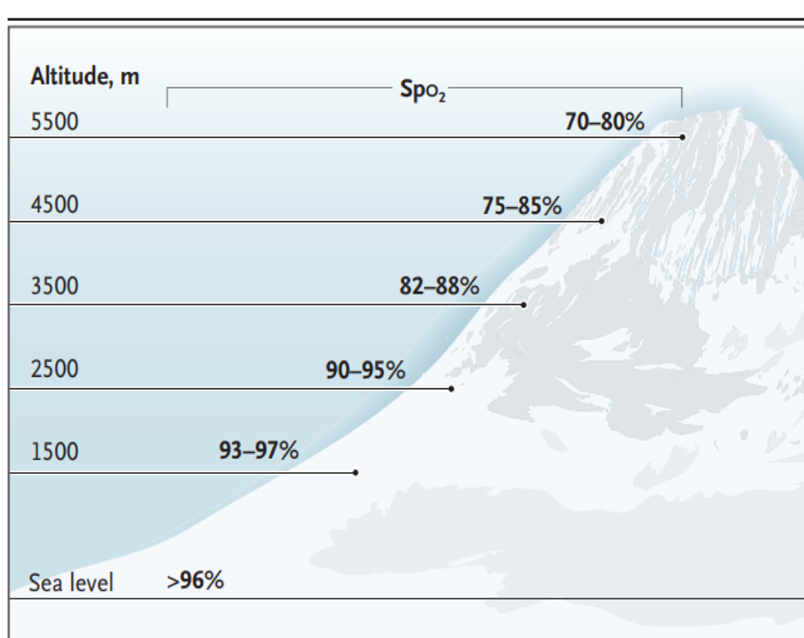
"From NEJM, A Luks and P Hackett, Medical conditions and high-altitude travel; 386(4):364–73. Copyright © (2022) Massachusetts Medical Society. Reprinted with permission."
Notes
Abbreviations: SpO2, oxygen saturation; m, meters.
Conversions: 1,500 m = ~4,900 ft; 2,500 m = ~8,200 ft; 3,500 m = ~11,500 ft; 4,500 m = ~14,750 ft; 5,500 m = ~18,000 ft
Treatment
AMS improves rapidly with a descent of 300 m (1,000 ft) or more, especially if exertion is minimal. If staying at the altitude of onset, supplemental oxygen at 1–2 L per minute will improve headache within about 30 minutes and resolve other AMS symptoms over hours, although it is rarely available. The popular small, handheld cans of compressed oxygen can provide brief relief but contain too little oxygen (5 L at most) for sustained improvement. Travelers with AMS but without HACE or HAPE (both described below) can remain safely at their current altitude and self-treat with non-opiate analgesics (e.g., ibuprofen 600 mg or acetaminophen 500 mg every 8 hours) and antiemetics (e.g., ondansetron 4 mg orally disintegrating tablets).
Acetazolamide speeds acclimatization and resolves AMS but is more commonly used and better validated for use as prophylaxis. Dexamethasone is more effective than acetazolamide at rapidly relieving the symptoms of moderate to severe AMS. If symptoms worsen while the traveler is at the same altitude and despite treatment, descent is mandatory.
High-altitude cerebral edema
As an encephalopathy, HACE is considered "end-stage" AMS. Fortunately, HACE is rare, especially at elevations <4,300 m (<14,000 ft). HACE is often a secondary consequence of the severe hypoxemia that occurs with HAPE.
Diagnosis
Unlike AMS, HACE presents with neurological findings, particularly altered mental status, ataxia, confusion, and drowsiness, similar to alcohol intoxication. Focal neurologic findings and seizures are rare in HACE; their presence should lead to suspicion of an intracranial lesion, a seizure disorder, or hyponatremia. Other considerations for the differential diagnosis include carbon monoxide poisoning, drug intoxication, hypoglycemia, hypothermia, and stroke. Coma can ensue within 24 hours of onset.
Treatment
In populated areas with access to medical care, HACE can be treated with supplemental oxygen and dexamethasone. In remote areas, initiate descent for anyone suspected of having HACE, in conjunction with dexamethasone and oxygen, if available. If descent is not feasible, supplemental oxygen or a portable hyperbaric device, in addition to dexamethasone, can be lifesaving. Coma is likely to ensue within 12–24 hours of the onset of ataxia in the absence of treatment or descent.
High-altitude pulmonary edema
HAPE can occur by itself or in conjunction with AMS and HACE; incidence is roughly 1 per 10,000 skiers in Colorado, and ≤1 per 100 travelers at >4,300 m (>14,000 ft).
Diagnosis
Early diagnosis is key; HAPE can be more rapidly fatal than HACE. Initial symptoms include chest congestion, cough, exaggerated dyspnea on exertion, and decreased exercise performance. If unrecognized and untreated, HAPE progresses to dyspnea at rest and frank respiratory distress, often with bloody sputum. This typical progression over 1–2 days is easily recognizable as HAPE, but the condition in those with poor ventilatory response may present only as central nervous system dysfunction, with confusion and drowsiness, while SpO2 is quite low.
Rales are detectable in most victims. Pulse oximetry can aid in making the diagnosis; oxygen saturation values of 50%–70% are common, which are at least 10 points lower than in healthy people at the same altitude. The differential diagnosis for HAPE includes bronchospasm, myocardial infarction, heart failure, pneumonia, and pulmonary embolism.
Treatment
In most circumstances, descent is urgent and mandatory. Administer oxygen, if available, and exert the patient as little as possible. If immediate descent is not an option, the use of supplemental oxygen or a portable hyperbaric chamber is critical.
Patients with mild HAPE who have access to oxygen (e.g., at a hospital or high-altitude medical clinic) might not need to descend to a lower altitude and can be treated with oxygen over 2–4 days and bedrest at the current altitude. In field settings, where resources are limited and the margin for error is lower, nifedipine can be used as an adjunct to descent, oxygen, or portable hyperbaric oxygen therapy. A selective phosphodiesterase inhibitor can be used if nifedipine is not available, but concurrent use of multiple pulmonary vasodilators is not recommended. Descent and oxygen are much more effective treatments than medication.
Sleep disturbance at high altitude
Sleep disturbance is the most common complaint of travelers to high altitudes. Although not necessarily associated with altitude illness, it can be bothersome. Above approximately 2,700 m (9,000 ft), some degree of periodic breathing becomes nearly universal and can interrupt sleep. In addition, sleep stage is altered and awakenings are frequent. Sleep generally but not always improves with acclimatization. Acetazolamide is effective for periodic breathing, and since it raises nocturnal SpO2, it can help with other aspects of altered sleep. Respiratory depressants such as alcohol and opiates should not be used to aid sleep at high altitude. Short half-life hypnotics, such as zolpidem 5 mg or zaleplon 5 mg, are recognized as generally safe and effective, but at least 8 hours after ingestion should be allowed for dissipation of effects before undertaking activities. Other agents such as diphenhydramine and melatonin have not been studied, but they do not depress the hypoxic ventilatory response and in small doses are considered safe.
Medications
Recommendations for use and dosages of medications to prevent and treat altitude illness are listed in Table 3.5.2.
Table 3.5.2: Recommended medication dosing to prevent and treat altitude illness
| Medication | Indication | Route | Dose |
|---|---|---|---|
| Acetazolamide1 | AMS, HACE prevention | PO |
125 mg twice a day; 250 mg twice a day if >100 kg body weight Pediatric: 1.25 mg/kg every 12 hours, up to 125 mg |
| AMS treatment | PO |
250 mg twice a day Pediatric 2.5 mg/kg every 12 hours, up to 250 mg |
|
| Dexamethasone2 | AMS, HACE prevention | PO |
2 mg every 6 hours or 4 mg every 12 hours Pediatric: not recommended for prophylaxis |
| AMS, HACE treatment | PO, IV, IM |
AMS: 4 mg every 6 hours HACE: 8 mg once, then 4 mg every 6 hours Pediatric: 0.15 mg/kg/dose every 6 hours up to 4 mg |
|
| Nifedipine2 | HAPE prevention | PO | 30 mg SR version every 12 hours or 20 mg SR version every 8 hours |
| HAPE treatment | PO | 30 mg SR version every 12 hours or 20 mg SR version every 8 hours | |
| Sildenafil2 | HAPE prevention | PO | 50 mg every 8 hours |
| Tadalafil2 | HAPE prevention | PO | 10 mg twice a day |
Notes
Abbreviations: AMS, acute mountain sickness; HACE, high-altitude cerebral edema; HAPE, high-altitude pulmonary edema; IM, intramuscular; IV, intravenous; PO, by mouth; SR, sustained release.
1FDA-approved for this use.
2Off-label for this use.
Acetazolamide
Mechanism of action
When taken preventively, acetazolamide hastens acclimatization to high-altitude hypoxia, thereby reducing the occurrence and severity of AMS. It also enhances recovery if taken after symptoms have developed. The drug works primarily by inducing bicarbonate diuresis and metabolic acidosis, which counteracts the respiratory alkalosis, thereby stimulating ventilation and increasing alveolar and arterial oxygenation, especially during sleep. By using acetazolamide, high-altitude ventilatory acclimatization that normally takes 3–5 days takes only 1 day.
Dose
An effective dose for prophylaxis that minimizes the common side effects of paresthesia is 125 mg every 12 hours, beginning the day before ascent and continuing the first 2 days at altitude, and longer if ascent continues. Acetazolamide can also be taken episodically for symptoms of AMS, as needed. To date, the only dose studied for treatment is 250 mg (2 doses taken 8 hours apart), although the lower dosage used for prevention has anecdotally been successful. The pediatric dose is 2.5 to 5 mg/kg/day in divided doses, up to 125 mg, twice a day.
Adverse and allergic reactions
Allergic reactions to acetazolamide are uncommon. Acetazolamide is a sulfonamide derivative, but cross-sensitivity between antimicrobial sulfonamides and acetazolamide, a non-antimicrobial sulfonamide, has not been reported. Thus, people allergic to sulfa antibiotics can take acetazolamide. However, a history of anaphylaxis to any medication, or a history of multiple drug allergies, requires caution. Although not an adverse reaction, acetazolamide inhibits carbonic anhydrase, which ordinarily catalyzes the breakdown of carbonic dioxide on the tongue when drinking carbonated drinks. This allows the person to taste the carbon dioxide on their tongue, altering the taste of the drink.
Dexamethasone
Dexamethasone is effective for preventing and treating AMS and HACE and might prevent HAPE as well. Unlike acetazolamide, if the drug is discontinued at altitude before acclimatization, mild rebound can occur. Acetazolamide is preferable to prevent AMS while ascending, and dexamethasone generally should be reserved for treatment, usually as an adjunct to descent. The adult dose is 4 mg every 6 hours; rarely is it needed for more than 1–2 days. An increasing trend is to use dexamethasone for "summit day" on high peaks (e.g., Aconcagua and Kilimanjaro) to prevent abrupt altitude illness.
Ibuprofen
Recent studies have shown that taking ibuprofen 600 mg every 8 hours helps prevent AMS, although not quite as effectively as acetazolamide. Ibuprofen is available over the counter, inexpensive, and well tolerated.
Nifedipine
Nifedipine both prevents and ameliorates HAPE. For prevention, nifedipine is generally reserved for people who are particularly susceptible to the condition. The adult dose for prevention or treatment is 30 mg of the sustained-release version every 12 hours or 20 mg every 8 hours. Nifedipine is difficult to dose in children, and amlodipine is preferred in children under 50 kg.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 inhibitors selectively lower pulmonary artery pressure, with less effect on systemic blood pressure than nifedipine. Tadalafil, 10 mg taken twice a day during ascent, can prevent HAPE. It is also being studied as a possible treatment.
Preventing severe altitude illness or death
The main point of instructing travelers about altitude illness is not to eliminate the possibility of mild illness but to prevent severe illness, need for evacuation, or death. Because the onset of symptoms and the clinical course are sufficiently slow and predictable, there is no reason for anyone to die from altitude illness unless they are trapped by weather or geography in situations where descent is impossible and treatment is inaccessible. Travelers can adhere to 3 rules to help prevent death or serious consequences from altitude illness:
- Know the early symptoms of altitude illness (same as a hangover) and be willing to acknowledge when symptoms are present.
- Never ascend to sleep at a higher altitude when experiencing symptoms of altitude illness, no matter how minor the symptoms seem.
- Descend if the symptoms become worse despite rest or treatment at the same elevation.
For trekking groups and expeditions going into remote high-altitude areas, where descent to a lower altitude could be problematic, a pressurization bag (e.g., the Gamow bag) can be beneficial. A foot pump produces an increased pressure of 0.14 kg/cm2 (2 lb/in2), mimicking a descent of approximately 1,500–1,800 m (5,000–6,000 ft) depending on the starting elevation. The total packed weight of the bag and pump is about 6.5 kg (14 lb).
Preexisting medical conditions
Travelers with preexisting medical conditions must optimize their treatment and have their conditions stable before departure (see Travelers with Chronic Illnesses chapter). In addition, these travelers should have plans for dealing with exacerbation of their conditions at high altitude. Travelers with underlying medical conditions (e.g., coronary artery disease, any form of chronic pulmonary disease or preexisting hypoxemia, obstructive sleep apnea [OSA], or sickle cell trait)—even if well-controlled—should consult a physician familiar with high-altitude medical issues before undertaking such travel (Table 3.5.3).
Healthcare professionals advising travelers should know that in most high-altitude resorts and cities, "home" oxygen is readily available, but in the United States, a prescription is required. Supplemental oxygen, whether continuous, episodic, or nocturnal, depending on the circumstances, is very effective at restoring oxygenation to low-altitude values and eliminates the risk of altitude illness and exacerbation of preexisting medical conditions.
Table 3.5.3: Ascent risk associated with various underlying medical conditions and risk factors
| Likely No Extra Risk | Caution Required | Contraindications |
|---|---|---|
|
|
|
Notes
Abbreviations: FEV1, forced expiratory volume in 1 second.
1Travelers with these conditions most often require consultation with a physician experienced in high-altitude medicine and a comprehensive management plan
2Class 1 obesity: Body mass index (BMI) of 30 to <35 kg/m2; Class 2 obesity: BMI of 35 to <40 kg/m2.
3Class 3 obesity: BMI of ≥40 kg/m2.
Diabetes mellitus
Travelers with diabetes can travel safely to high altitudes, but they must be accustomed to exercise if participating in strenuous activities and should carefully monitor their blood glucose. Diabetic ketoacidosis can be triggered by altitude illness and can be more difficult to treat in people taking acetazolamide. Not all glucometers read accurately at high altitude.
Obstructive sleep apnea
Travelers with sleep disordered breathing who are planning high-altitude travel should receive acetazolamide. Those with mild to moderate OSA who are not hypoxic at home might do well without a continuous positive airway pressure (CPAP) device, while those with severe OSA should be advised to avoid high-altitude travel unless they receive supplemental oxygen in addition to their CPAP. Oral appliances for OSA can be useful adjuncts when electrical power is unavailable.
Pregnancy
There are no studies or case reports describing fetal harm among women who briefly travel to high altitude during their pregnancy. Nevertheless, healthcare professionals might be prudent to recommend that pregnant women do not stay at sleeping altitudes >3,050 m (>10,000 ft). Travel to high altitudes during pregnancy warrants confirmation of good maternal health and verification of a low-risk gestation. Advise pregnant travelers of the dangers of having a pregnancy complication in remote, mountainous terrain.
Radial keratotomy
Most people do not have visual problems at high altitude. At very high altitudes, however, some people who have had radial keratotomy procedures might develop acute farsightedness and be unable to care for themselves. Laser-assisted in situ keratomileusis (LASIK) and other newer procedures may produce only minor visual disturbances at high elevations.
- Bärtsch, P., & Swenson, E. R. (2013). Acute high-altitude illnesses. The New England Journal of Medicine, 369(17), 1666–1667. https://www.doi.org/10.1056/NEJMc1309747
- Hackett, P. H., Luks, A. M., Lawley, J. S., & Roach, R. C. (2017). Wilderness medicine, high-altitude medicine and pathophysiology (7th ed., pp. 8–28). Elsevier.
- Hackett, P. H., & Roach, R. C. (2004). High altitude cerebral edema. High Altitude Medicine & Biology, 5(2), 136–146. https://www.doi.org/10.1089/1527029041352054
- Luks, A. M., Beidleman, B. A., Freer, L., Grissom, C. K., Keyes, L. E., McIntosh, S. E., . . . Hackett, P. H. (2023). Wilderness Medical Society clinical practice guidelines for the prevention, diagnosis, and treatment of acute altitude illness: 2024 update. Wilderness & Environmental Medicine, 35(1), 2S–19S. https://www.doi.org/10.1016/j.wem.2023.05.013
- Luks, A. M., & Hackett, P. H. (2022). Medical conditions and high-altitude travel. The New England Journal of Medicine, 386(4), 364–373. https://www.doi.org/10.1056/NEJMra2104829
- Luks, A. M., & Swenson, E. R. (2008). Medication and dosage considerations in the prophylaxis and treatment of high-altitude illness. Chest, 133(3), 744–755. https://www.doi.org/10.1378/chest.07-1417
- Meier, D., Collet, T. H., Locatelli, I., Cornuz, J., Kayser, B., Simel, D. L., & Sartori, C. (2017). Does this patient have acute mountain sickness? The rational clinical examination systematic review. JAMA, 318(18), 1810–1819. https://www.doi.org/10.1001/jama.2017.16192
- Roach, R. C., Lawley, J. S., & Hackett, P. H. (2017). Wilderness medicine, high-altitude physiology (7th ed., pp. 2–8). Elsevier.
- Shlim, D. R. (2020). The use of acetazolamide for the prevention of high-altitude illness. Journal of Travel Medicine, 27(6), 1–6. https://www.doi.org/10.1093/jtm/taz106
- Woolcott, O. O. (2021). The Lake Louise acute mountain sickness score: Still a headache. High Altitude Medicine & Biology, 22(4), 351–352. https://www.doi.org/10.1089/ham.2021.0110
