Purpose

Introduction
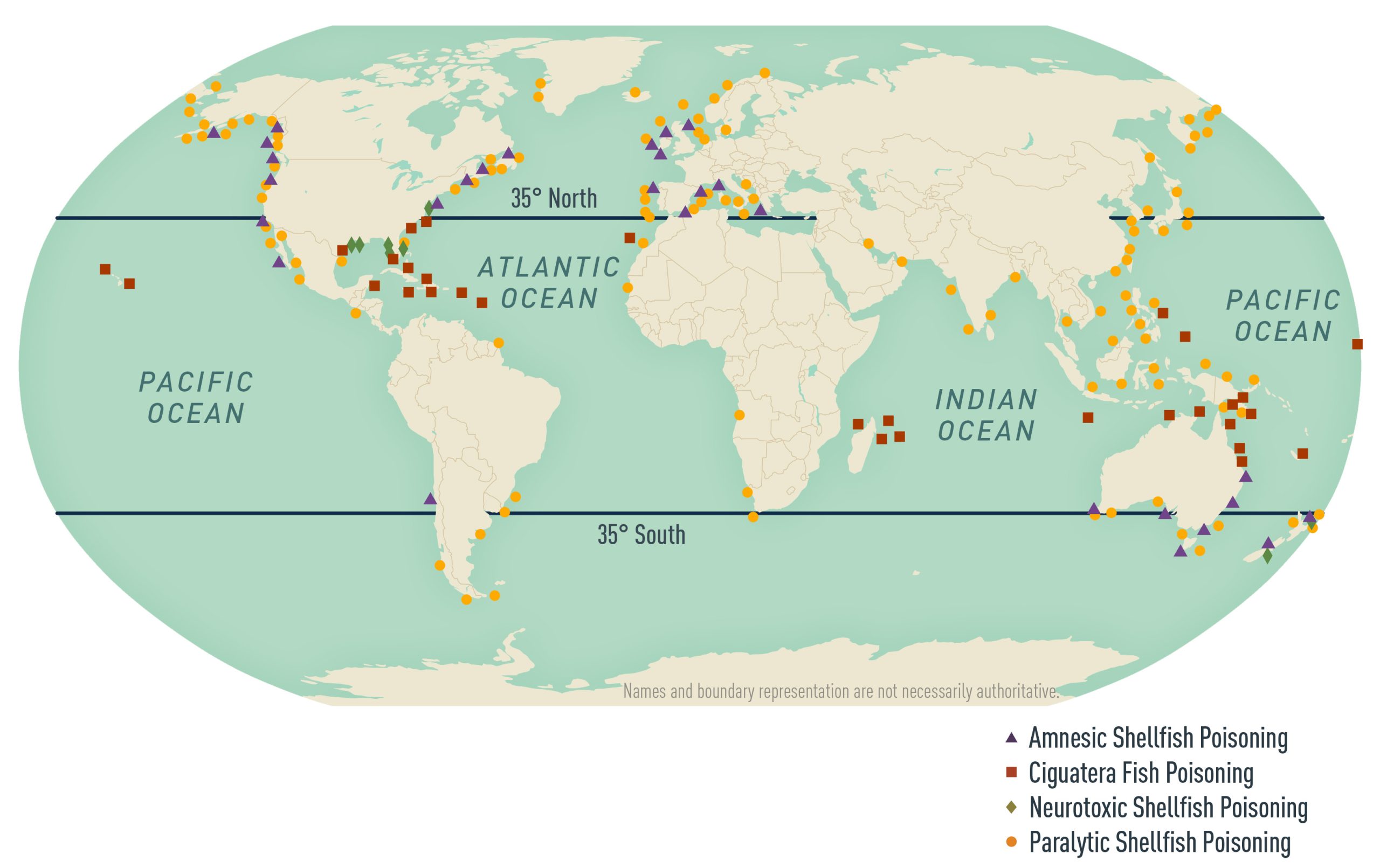
Poisoning from ingesting marine toxins is an underrecognized hazard for travelers, particularly in the tropics and subtropics (Table 3.8.1). Climate change, coral reef damage, expanding international trade and tourism, growing seafood consumption, and spread of toxic algal blooms are all contributing to an increasing risk (Map 3.8.1).
Table 3.8.1: Diseases caused by marine toxins
| Disease - Paralytic shellfish poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Saxitoxin | Karenia brevis | Bivalve molluscs, particularly clams and mussels | Worldwide, most common in temperate coastal waters | Most common and most severe form of shellfish poisoning; neurologic symptoms with gastroenteritis; severe manifestations include dizziness, difficulty swallowing, weakness, and/or respiratory failure | 30 minutes to 4 hours |
| Disease - Diarrheic shellfish poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Okadaic acid | Dinophysis species | Bivalve molluscs | Worldwide | Gastroenteritis | 30 minutes to 2 hours |
| Disease - Neurotoxic shellfish poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Brevetoxins | Karenia brevis | Bivalve molluscs | Rare, most common in temperate coastal waters, especially U.S. Northeast, Pacific Northwest, and Alaska | Gastroenteritis followed by neurologic illness (resembles mild ciguatera or paralytic shellfish poisoning); bronchoconstriction following inhalation of aerosolized brevetoxins in sea spray (aerosolized red tide respiratory irritation) | 30 minutes to 3 hours |
| Disease - Scombroid (histamine) fish poisoning | ||||||
|---|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset | |
| Histamine | Histidine converted to histamine and other scombrotoxins by bacterial action | Inadequately chilled histidine-rich fish (e.g., mahi-mahi, tuna, mackerel, skipjack) | Worldwide | Resembles an acute allergic reaction: flushing, headache, urticaria, wheezing, and gastroenteritis | 10–60 minutes | |
| Disease - Amnesic shellfish poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Domoic acid | Pseudo-nitzschia species | Mussels, razor clams, crustaceans | Extremely rare; outbreaks in North America, Europe, New Zealand, and Australia | Gastroenteritis followed by neurologic illness causing cognitive impairment, retrograde or antegrade amnesia; severe illness is rare | Gastroenteritis symptoms in <24 hours; neurologic illness in <48 hours |
| Disease - Ciguatera fish poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Ciguatoxin, maitotoxin | Gambierdiscus toxicus and other dinoflagellates | Large carnivorous tropical subtropical reef fish (e.g., barracuda, grouper, snapper, amberjack, moray eel, sea bass) | Tropical and subtropical waters between 25°N and 35°S; most common in the Pacific Ocean, Indian Ocean, and Caribbean Sea | Gastroenteritis followed by multiple neurological signs and symptoms; cardiovascular signs include bradycardia and hypotension | Gastrointestinal 1–6 hours; neurological symptoms delayed for up to 96 hours and may last for months or years |
| Disease - Pufferfish (fugu) poisoning | |||||
|---|---|---|---|---|---|
| Toxin | Origin of Toxin | Seafood Vehicle | Geographic Distribution | Typical Symptoms | Typical Onset |
| Tetrodotoxin | Bacterial synthesis | Pufferfish, porcupine fish, ocean sunfish, horseshoe crabs | Worldwide, most common in Japan and Indo-Pacific Ocean | Paresthesia, dizziness, paralysis | 10 minutes to 4 hours |
Map 3.8.1
Centers for Disease Control and Prevention
Ciguatera fish poisoning
Ciguatera fish poisoning occurs after eating reef fish contaminated with toxins such as ciguatoxin or maitotoxin. These potent toxins originate from Gambierdiscus toxicus, a small marine organism (dinoflagellate) that grows on and around coral reefs. Dinoflagellates are eaten by herbivorous fish. The toxins produced by G. toxicus are then modified and concentrated as they pass up the marine food chain to carnivorous fish and finally to humans. Ciguatoxins are concentrated in fish liver, intestines, roe, and heads.
G. toxicus might proliferate on dead coral reefs more effectively than other dinoflagellates. The risk for ciguatera poisoning is likely to increase as coral reefs deteriorate because of climate change, ocean acidification, offshore construction, and nutrient runoff.
Risk to travelers
Approximately 50,000 cases of ciguatera poisoning are reported worldwide annually, but because the disease is underrecognized and underreported, reports are likely grossly underestimated. The incidence in travelers to highly endemic areas has been estimated as high as 3 per 100. Ciguatera is widespread in tropical and subtropical waters, usually between the latitudes of 35°N and 35°S, and is particularly common in the Pacific and Indian Oceans and the Caribbean Sea. The incidence and geographic distribution of ciguatera poisoning are increasing. Newly recognized areas of risk include Madeira, the Canary Islands, and the western Gulf of America. Be aware that travelers with ciguatera fish poisoning might seek care after returning home to non-endemic (temperate) areas. In addition, cases of ciguatera fish poisoning are seen with increasing frequency in non-endemic areas as a result of the increasing global trade in seafood products.
Fish most likely to cause ciguatera poisoning are large carnivorous reef fish (e.g., amberjack, barracuda, grouper, moray eel, sea bass, sturgeon). Omnivorous and herbivorous fish (e.g., parrot fish, red snapper, surgeonfish) can also be a risk.
Clinical presentation
Ciguatera poisoning can cause gastrointestinal, neurologic, neuropsychiatric, and cardiovascular symptoms. The first symptoms usually develop within 3–6 hours after eating contaminated fish but can be delayed up to 30 hours. Typically, there is a gastrointestinal illness with diarrhea, nausea, vomiting, and abdominal pain, accompanied by or closely followed by a wide range of neurological and neuropsychiatric signs. These include paresthesia, weakness, pain in the teeth or a sensation that the teeth are loose, a burning or metallic taste in the mouth, impaired memory, depression, chronic fatigue, generalized itching, sweating, and blurred vision. Cold allodynia (abnormal sensation when touching cold water or objects) and temperature reversal (cold items feel hot; hot items feel cold) are pathognomonic but not always present. Neurologic features usually last a few days to several weeks but can persist for months or even years. Cardiovascular signs and symptoms include bradycardia, heart block, or hypotension.
The death rate from ciguatera poisoning is <0.1% but varies according to the toxin dose and availability of medical care to deal with complications. The diagnosis of ciguatera poisoning is based on the characteristic signs and symptoms and a history of eating fish species known to carry ciguatera toxin. No specific diagnostic tests to detect ciguatoxins are currently available.
Prevention
Ciguatera toxins do not affect the texture, taste, or smell of fish, and they are not destroyed by gastric acid or by canning, cooking, freezing, pickling, salting, or smoking. To prevent ciguatera fish poisoning, travelers should avoid consumption of reef fish, particularly fish that weigh >5 pounds; counsel travelers to never eat high-risk fish (e.g., barracuda, moray eel) and to avoid eating the parts of the fish (e.g., the head, intestines, liver, roe) that concentrate ciguatera toxin.
Treatment
No specific antidote for ciguatoxin or maitotoxin poisoning is available. Symptomatic treatments include amitriptyline for chronic paresthesia and depression; fluoxetine for chronic fatigue; gabapentin or pregabalin for neuropathic symptoms; nifedipine or acetaminophen for headaches: and antihistamines for pruritus. Intravenous mannitol has been reported in uncontrolled studies to reduce the severity and duration of neurologic symptoms, particularly if given ≤48 hours of symptom onset; give mannitol only to hemodynamically stable, well-hydrated patients. For patients who are not vomiting, consider administering activated charcoal. For patients with significant gastrointestinal fluid losses, initiate intravenous fluids and replenish electrolytes. Orthostatic hypotension typically responds to intravenous fluids and alpha-adrenergic agonists. Patients with symptomatic bradycardia might need atropine. After recovery, advise patients to avoid consuming alcohol, caffeine, fish, and nuts for ≥6 months because these might cause symptom relapse.
Pufferfish (fugu) poisoning
Pufferfish poisoning occurs after ingestion of fish containing tetrodotoxin, a potent neurotoxin. Potentially toxic fish are distributed widely worldwide and include pufferfish, porcupine fish, and ocean sunfish. The toxin is usually concentrated in the ovaries, liver, intestine, and skin of the fish. Most cases of pufferfish (fugu) poisoning occur in Japan, where it is served raw as fugu, a highly prized delicacy. Tetrodotoxin has also been found in other marine animals such as horseshoe crabs, mollusks, blue-ringed octopus, and moon snails. Poisoning has been reported following consumption of horseshoe crab eggs in Thailand and Malaysia. Rare cases of tetrodotoxin poisoning have occurred in the United States.
Clinical presentation
Symptoms of pufferfish poisoning may develop within 10 minutes of ingestion of toxic fish or may be delayed for more than 4 hours. Early symptoms typically include paresthesias and numbness of the lips and mouth, followed by nausea and dizziness. Later, there may be headache, generalized paresthesias and numbness, ascending paralysis, hypersalivation, diaphoresis, vomiting, abdominal pain, and diarrhea. In the most severe cases there is widespread paralysis, respiratory failure, bradycardia and other arrhythmias, and circulatory collapse.
Prevention
Tetrodotoxin is a highly potent neurotoxin that is not destroyed by gastric acid or by canning, cooking, freezing, pickling, salting, or smoking. In view of the potentially serious nature of tetrodotoxin poisoning, travelers should be advised to avoid any potentially toxic fish, even when prepared by trained chefs in licensed restaurants.
Treatment
There is no specific antidote for tetrodotoxin, and treatment is designed to limit absorption of toxin and provide supportive care. Gastric lavage and activated charcoal may be indicated to limit absorption of toxin. All patients should be admitted to a hospital for observation. In moderate or severe cases, patients should be admitted to an intensive care unit. Intravenous fluids, vasopressors, endotracheal intubation, and respiratory support may be necessary. Bradycardia may respond to atropine.
Scombroid poisoning
Scombroid is caused by eating fish that contain high levels of histamine and other scombrotoxins. As a result of improper handling and storage, histidine is converted to histamine and other scombrotoxins by bacteria with high histidine decarboxylase activity. Conversion of histidine to histamine and other scombrotoxins occurs optimally at 20°C–30°C (68°F–86°F), which typically occurs in fish that have not been promptly refrigerated or frozen after capture.
Risk to travelers
Scombroid occurs worldwide in both temperate and tropical waters and is one of the most common fish poisonings. Fish typically associated with scombroid have naturally high levels of histidine in their flesh and include amberjack, anchovies, bluefish, herring, mackerel, mahi mahi (dolphin fish), marlin, sardines, and tuna. After histidine is converted to histamine and other scombrotoxins, it is not destroyed by gastric acid or by canning, cooking, freezing, pickling, salting, or smoking.
Clinical presentation
Scombroid poisoning resembles an acute allergic reaction and usually appears 10–60 minutes after a person eats contaminated fish. Signs and symptoms include abdominal cramps and diarrhea, blurred vision, flushing of the face and upper body resembling sunburn, severe headaches, itching, and palpitations. Left untreated, symptoms usually resolve within 12 hours but can last ≤48 hours.
Rarely, respiratory compromise, malignant arrhythmias, and hypotension requiring hospitalization can occur. Scombroid poisoning has no long-term sequelae and usually is diagnosed from clinical signs and symptoms. Clustering of cases helps exclude the possibility of true fish allergy.
Prevention
Fish contaminated with histamine can have a peppery, sharp, or salty taste or a "bubbly" feel, but will usually look, smell, and taste normal. The key to prevention is to make sure fish are properly iced or refrigerated at temperatures <3.3°C (<38°F) or immediately frozen after being caught.
Treatment
Scombroid poisoning usually responds well to antihistamines, typically H1-receptor antagonists, although H2-receptor antagonists also might provide some benefit.
Shellfish poisoning
Shellfish, including crustaceans (Dungeness crab, lobster, and shrimp), filter-feeding bivalve mollusks (clams, cockles, mussels, oysters, and scallops), and gastropod mollusks (abalone, moon snails, and whelks) can harbor toxins that result in several different poisoning syndromes. Toxins originate in small marine organisms (diatoms or dinoflagellates) ingested and concentrated by shellfish.
Risk to travelers
Contaminated (toxic) shellfish can be found in temperate and tropical waters, typically during or after phytoplankton blooms, also called harmful algal blooms. One example of a harmful algal bloom is the Florida red tide caused by Karenia brevis.
Clinical presentation
Poisoning results in gastrointestinal and neurologic illness of varying severity. Symptoms typically appear 30–60 minutes after a person ingests toxic shellfish but can be delayed for several hours. Diagnosis is usually made by exclusion and typically is made clinically in patients with a history of having recently eaten shellfish.
Amnesic shellfish poisoning
Amnesic shellfish poisoning is a rare form of shellfish poisoning caused by eating shellfish contaminated with domoic acid, produced by diatoms of the Pseudo-nitzschia spp. Outbreaks of amnesic shellfish poisoning have been reported in the Americas (Canada and Chile), Europe (Belgium, France, Ireland, Portugal, Scotland, and Spain), and the Pacific (Australia and New Zealand). Implicated shellfish include razor clams, mussels, scallops, and other crustaceans.
In most cases, gastrointestinal symptoms (e.g., abdominal pain, diarrhea, vomiting) develop within 24 hours of eating toxic shellfish, followed by headache, cognitive impairment, and memory loss. Symptoms usually resolve within hours to days after shellfish ingestion. Hypotension, arrhythmias, ophthalmoplegia, coma, and death have been reported in severe cases. Survivors might exhibit severe anterograde, short-term memory deficits.
Diarrheic shellfish poisoning
Diarrheic shellfish poisoning results from eating shellfish contaminated with toxins (e.g., okadaic acid). Diarrheic shellfish poisoning occurs worldwide, and outbreaks have been reported in the Americas (Canada, Chile, United States, and Uruguay), Asia (China and Japan), and Europe (Belgium, France, Ireland, Scandinavia, and Spain).
Most cases result from eating toxin-containing bivalve mollusks (e.g., mussels, scallops). Symptoms usually occur within 2 hours of consumption and include abdominal pain, chills, diarrhea, nausea, and vomiting. Symptoms usually resolve within 2–3 days. No deaths from diarrheic shellfish poisoning have been reported.
Neurotoxic shellfish poisoning
Neurotoxic shellfish poisoning is caused by eating shellfish contaminated with brevetoxins produced by the dinoflagellate K. brevis. Neurotoxic shellfish poisoning is predominantly an illness of the Western Hemisphere (the Caribbean, Gulf of America, southeastern coast of the United States), but the disease also has been reported from New Zealand.
Neurotoxic shellfish poisoning usually presents as a gastroenteritis accompanied by neurologic symptoms resembling mild ciguatera or paralytic shellfish poisoning (described below), 30 minutes to 3 hours after a person eats shellfish. Aerosolized red tide respiratory irritation also can occur when people inhale aerosolized brevetoxins in sea spray and has been reported in association with a red tide (K. brevis harmful algal bloom) in Florida. Aerosolized red tide respiratory irritation can induce bronchoconstriction and cause acute, temporary respiratory discomfort in healthy people. People with asthma might experience more severe and prolonged respiratory effects.
Paralytic shellfish poisoning
Paralytic shellfish poisoning (PSP) is the most common and most severe form of shellfish poisoning. PSP is caused by eating shellfish contaminated with saxitoxins. These potent neurotoxins are produced by various dinoflagellates. A wide range of shellfish can cause PSP, but most cases occur after people eat clams or mussels.
PSP occurs worldwide but is most common in temperate waters off the Atlantic and Pacific coasts of North America, including Alaska. Other countries in the Americas (Chile), as well as countries in Asia (China and the Philippines), Europe (Ireland and Scotland), and the Pacific (Australia and New Zealand), have also reported cases.
Symptoms usually appear 30–60 minutes after a person eats toxic shellfish and include numbness and tingling of the face, lips, tongue, arms, and legs. Patients also might have diarrhea, vomiting, headache, and nausea. Severe cases are associated with ingestion of large doses of toxin and clinical features such as ataxia, dysphagia, flaccid paralysis, mental status changes, and respiratory failure. The case-fatality ratio depends on the availability of modern medical care, including mechanical ventilation; rates of death among children can be particularly high.
Prevention
Shellfish poisoning can be prevented by avoiding potentially contaminated shellfish, which is particularly important in areas during or shortly after algal blooms, locally referred to as "red tides" or "brown tides." Consuming shellfish also carries a very high risk for infection from various viral (e.g., hepatitis A virus, norovirus) and bacterial (e.g., Salmonella, Shigella, Vibrio parahaemolyticus, and Vibrio vulnificus) pathogens. Ideally, travelers to developing countries should avoid eating shellfish. Marine shellfish toxins cannot be destroyed by gastric acid or by canning, cooking, freezing, pickling, salting, or smoking.
Treatment
Treatment is symptomatic and supportive. Severe cases of paralytic shellfish poisoning might require mechanical ventilation.
- Chinain, M., Gatti, C. M. I., Darius, H. T., Quod, J.-P., & Tester, P. A. (2021). Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae, 102, 1–22. https://www.doi.org/10.1016/j.hal.2020.101873
- Friedman, M. A., Fleming, L. E., Fernandez, M., Bienfang, P., Schrank, K., Dickey, R., . . . Reich, A. (2008). Ciguatera fish poisoning: Treatment, prevention and management. Marine Drugs, 6(3), 456–479. https://www.doi.org/10.3390/md20080022
- Gouel, P., Gatti, C. M. I., de Haro, L., Liautaud, A., Langrand, J., & Boucaud-Maitre, D. (2022). Tetrodotoxin poisoning in mainland France and French overseas territories: A review of published and unpublished cases. Toxins, 14(5), 351. https://www.doi.org/10.3390/toxins14050351
- Hungerford, J. M. (2021). Histamine and scombrotoxins. Toxicon: Official Journal of the International Society on Toxinology, 201, 115–126. https://www.doi.org/10.1016/j.toxicon.2021.08.013
- Isbister, G. K., & Kiernan, M. C. (2005). Neurotoxic marine poisoning. The Lancet: Neurology, 4(4), 219–228. https://www.doi.org/10.1016/S1474-4422(05)70041-7
- Loeffler, C. R., Tartaglione, L., Friedemann, M., Spielmeyer, A., Kappenstein, O., & Bodi, D. (2021). Ciguatera mini review: 21st century environmental challenges and the interdisciplinary research efforts rising to meet them. International Journal of Environmental Research and Public Health, 18(6), 3027. https://www.doi.org/10.3390/ijerph18063027
- Raposo, M. I. C., Gomes, M. T. S. R., Botelho, M. J., & Rudnitskaya, A. (2020). Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxins, 12(5), 344. https://www.doi.org/10.3390/toxins12050344
- Résière, D., Florentin, J., Mehdaoui, H., Mahl, Z., Gueye, P., Hommel, D., . . . Mégarbane, B. (2022). Clinical characteristics of ciguatera poisoning in Martinique, French West Indies: A case series. Toxins, 14(8), 535. https://www.doi.org/10.3390/toxins14080535
