
Toxoplasmosis
[Toxoplasma gondii]
Causal Agents
Toxoplasma gondii is a protozoan parasite that infects most species of warm-blooded animals, including humans, and causes the disease toxoplasmosis.
Life Cycle
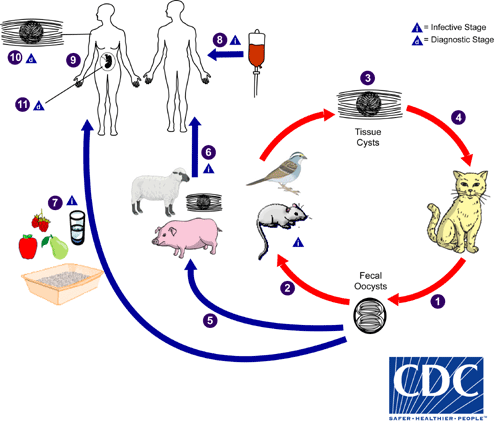
The only known definitive hosts for Toxoplasma gondii are members of family Felidae (domestic cats and their relatives). Unsporulated oocysts are shed in the cat’s feces  . Although oocysts are usually only shed for 1–3 weeks, large numbers may be shed. Oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds and rodents) become infected after ingesting soil, water or plant material contaminated with oocysts
. Although oocysts are usually only shed for 1–3 weeks, large numbers may be shed. Oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds and rodents) become infected after ingesting soil, water or plant material contaminated with oocysts  . Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites
. Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites  . Cats become infected after consuming intermediate hosts harboring tissue cysts
. Cats become infected after consuming intermediate hosts harboring tissue cysts  . Cats may also become infected directly by ingestion of sporulated oocysts. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment
. Cats may also become infected directly by ingestion of sporulated oocysts. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment  . Humans can become infected by any of several routes:
. Humans can become infected by any of several routes:
- Eating undercooked meat of animals harboring tissue cysts
 .
. - Consuming food or water contaminated with cat feces or by contaminated environmental samples (such as fecal-contaminated soil or changing the litter box of a pet cat)
 .
. - Blood transfusion or organ transplantation
 .
. - Transplacentally from mother to fetus
 .
.
In the human host, the parasites form tissue cysts, most commonly in skeletal muscle, myocardium, brain, and eyes; these cysts may remain throughout the life of the host. Diagnosis is usually achieved by serology, although tissue cysts may be observed in stained biopsy specimens  . Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR
. Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR  .
.
Geographic Distribution
Serologic prevalence data indicate that toxoplasmosis is one of the most common human infections throughout the world. A high prevalence of infection in France has been related to a preference for eating raw or undercooked meat, while a high prevalence in Central America has been related to the frequency of stray cats in a climate favoring survival of oocysts and soil exposure. The overall seroprevalence in the United States among adolescents and adults, as determined with specimens collected by the third National Health and Nutrition Examination Survey (NHANES III) between 1988 and 1994, was found to be 22.5%, with a seroprevalence among women of childbearing age (15 to 44 years) of 15%. In a more recent evaluation using data from NHANES 2009–2010, the overall age-adjusted T. gondii antibody seroprevalence among persons > 6 years of age was 12.4%, and among women 15–44 years of age was 9.1%.
Clinical Presentation
Acquired infection with Toxoplasma in immunocompetent persons is generally an asymptomatic infection. However, 10% to 20% of patients with acute infection may develop cervical lymphadenopathy and/or a flu-like illness. The clinical course is usually benign and self-limited; symptoms usually resolve within a few weeks to months. In rare cases ocular infection with visual loss can occur. Immunodeficient patients often have central nervous system (CNS) disease but may have retinochoroiditis, pneumonitis, or other systemic disease. In patients with AIDS, toxoplasmic encephalitis is the most common cause of intracerebral mass lesions and is thought to usually be caused by reactivation of chronic infection. Toxoplasmosis in patients being treated with immunosuppressive drugs may be due to either newly acquired or reactivated latent infection.
Congenital toxoplasmosis results from an acute primary infection acquired by the mother during pregnancy. The incidence and severity of congenital toxoplasmosis vary with the trimester during which infection was acquired. Because treatment of the mother may reduce the incidence of congenital infection and reduce sequelae in the infant, prompt and accurate diagnosis is important. Many infants with subclinical infection at birth will subsequently develop signs or symptoms of congenital toxoplasmosis. Ocular Toxoplasma infection, an important cause of retinochoroiditis in the United States, can be the result of congenital infection, or infection after birth. In congenital infection, patients are often asymptomatic until the second or third decade of life, when lesions develop in the eye.
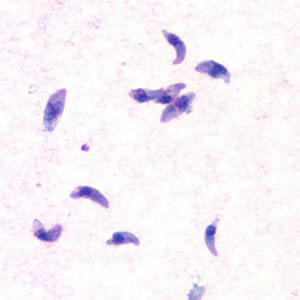
Toxoplasma gondii tachyzoites.
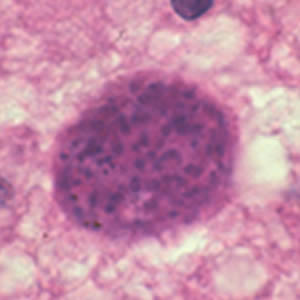
Toxoplasma gondii cyst in brain tissue.

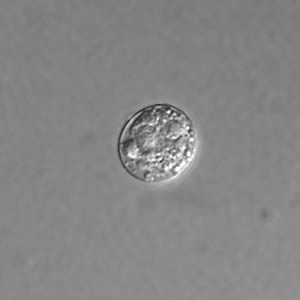
Toxoplasma gondii cyst, unstained.
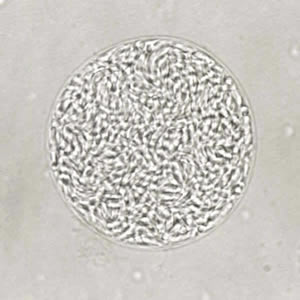
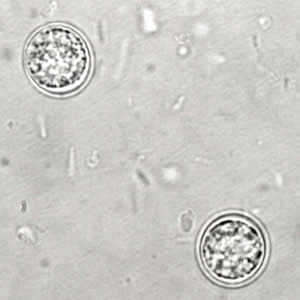
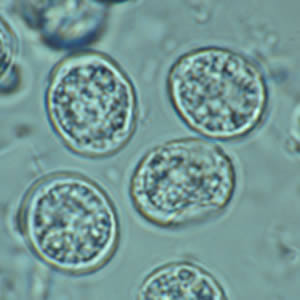
Toxoplasma gondii sporulated oocyst.
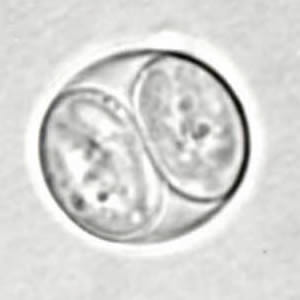
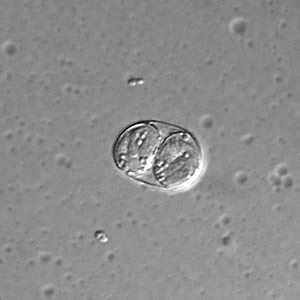
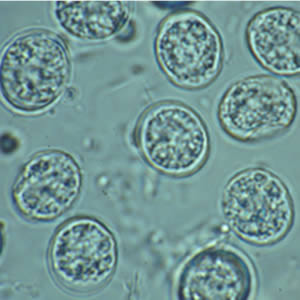
Toxoplasma gondii unsporulated oocysts.
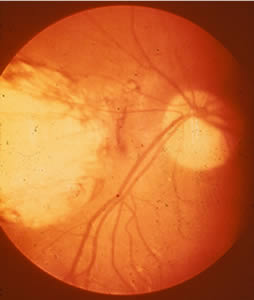
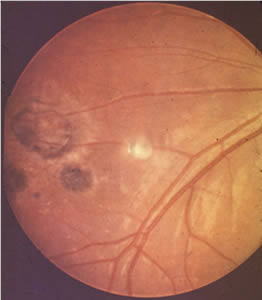
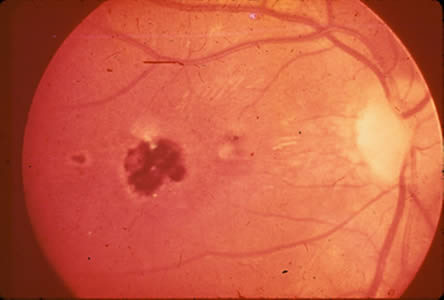
Ocular toxoplasmosis: Chorioretinitis.
Laboratory Diagnosis
The diagnosis of toxoplasmosis may be documented by:
- Serologic testing, which is the routine method of diagnosis.
- Observation of parasites in patient specimens, such as bronchoalveolar lavage material from immunocompromised patients, or lymph node biopsy.
- Detection of parasite genetic material by PCR, especially in detecting congenital infections in utero and toxoplasmic encephalitis in people with HIV.
- The use of other methods, for example isolation of parasites from blood or other body fluids by intraperitoneal inoculation into mice or tissue culture, are rarely performed.
Antibody Detection
The detection of Toxoplasma-specific antibodies is the primary diagnostic method to determine infection with Toxoplasma. Toxoplasma antibody detection tests are performed by a large number of laboratories with commercially available kits. The most widely available are tests for detection of IgG and IgM antibodies.
Persons should be initially tested for the presence of Toxoplasma-specific IgG and IgM antibodies to determine their infection status. A positive IgG titer indicates infection with the organism at some time. A negative result on an IgM test essentially excludes recent infection, but a positive IgM test result is difficult to interpret because Toxoplasma-specific IgM antibodies may be detected for as long as 18 months after newly acquired infection. The timing of testing is also an important consideration when interpreting the results. For example, a positive IgG and negative IgM result when testing is performed late in gestation could represent an infection acquired in the distant past. Alternatively, it could represent an infection acquired during early pregnancy and the IgM has already peaked by the time of testing, in which case the fetus could be at risk for congenital infection.
The sensitivity and specificity of these assays, especially IgM tests, can vary by methodology and laboratory; when combined with the prolonged retention of IgM antibodies among some people, the IgM results may be easily misinterpreted. In situations where determining the timing of infection is critical, for example in pregnant women, follow up or confirmatory testing is recommended. Confirmatory testing involves performing a panel of serological tests that measure different antibodies that rise and fall at different times during infection; these results are then interpreted in combination with one another to determine when a patient was likely infected. This type of testing is available at the Jack S. Remington Laboratory for Specialty Diagnostics at Sutter Health in Palo Alto, California.
Newborn infants suspected of congenital toxoplasmosis should be tested by IgG, IgM, and IgA. Toxoplasma-specific IgA tests may be available at some commercial laboratories. IgM and IgA antibodies do not cross the placenta and reflect the infant’s immune response; however, their detection before 5 and 10 days of age, respectively, could reflect cross contamination with the mother’s blood. False negative results can occur in cases where fetal infection was acquired very early in gestation. Consequently, all specimens from neonates suspected of having congenital toxoplasmosis should be sent to the Jack S. Remington Laboratory for Specialty Diagnostics at Sutter Health in Palo Alto, California, which has the most experience with infant testing.
People with HIV and active toxoplasmosis of the central nervous system are often seropositive for Toxoplasma-specific IgG antibodies. The absence of Toxoplasma-specific IgG antibodies does not rule out toxoplasmosis but makes it less likely. Tests for IgM antibodies are generally negative. Quantitative antibody titers are not useful for diagnosis.
References:
References:
- NCCLS. Clinical Use and Interpretation of Serologic Tests for Toxoplasma gondii; Approved Guideline. NCCLS document M36-A [ISBN 1-56238-523-2]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898 USA, 2004.
- McAuley JM, Jones JL, Singh AM. Toxoplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington, D.C.: American Society for Microbiology; 2015. p. 2373–2386.
- Remington JS, McLeod R, Wilson CB, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia, PA: The WB Saunders Co.; 2011. p. 918-1041.
- Dhakal R, Gajurel K, Pomares C, Talucod J, Press CJ, Montoya JG. Significance of a positive Toxoplasma immunoglobulin M test result in the United States. J Clin Microbiol 2015;53:3601–3605. doi:10.1128/JCM.01663-15.
- Maldonado YA, Read JS, AAP COMMITTEE ON INFECTIOUS DISEASES. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 2017;139(2):e20163860.
- Guidelines for the Prevention and Treatment of Opportunistic Infections on Adults and Adolescents with HIV: Toxoplasmosis. Available at Toxoplasma gondii Encephalitis: Adult and Adolescent OIs | NIH. Accessed 4/8/2025.
Treatment Information
Treatment information for toxoplasmosis can be found at:
Clinical Care of Toxoplasmosis
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.